Effects of hypocretin2-saporin and antidopamine-beta-hydroxylase-saporin neurotoxic lesions of the dorsolateral pons on sleep and muscle tone
- PMID: 15147308
- PMCID: PMC1201541
- DOI: 10.1111/j.0953-816X.2004.03366.x
Effects of hypocretin2-saporin and antidopamine-beta-hydroxylase-saporin neurotoxic lesions of the dorsolateral pons on sleep and muscle tone
Abstract
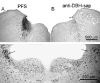
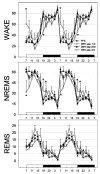
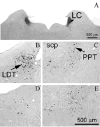
The hypocretin neurons have been implicated in regulating sleep-wake states as they are lost in patients with the sleep disorder narcolepsy. Hypocretin (HCRT) neurons are located only in the perifornical region of the posterior hypothalamus and heavily innervate pontine brainstem neurons, such as the locus coeruleus (LC), which have traditionally been implicated in promoting arousal. It is not known how the hypocretin innervation of the pons regulates sleep-wake states as pontine lesions have never been shown to increase sleep. It is likely that in previous studies specific neurons were not lesioned. Therefore, in this study, we applied saporin-based neurotoxins to the dorsolateral pons and monitored sleep in rats. Anti-dopamine-beta-hydroxylase-saporin killed the LC neurons but sleep was affected only during a two hour light-dark transition period. Application of hypocretin2-saporin killed fewer LC neurons relative to other adjacent neurons. This occurred because the LC neurons possess the hypocretin receptor 1 but the ligand hypocretin 2 binds to this receptor with less affinity relative to the hypocretin receptor 2. The hypocretin2-saporin lesioned rats compared to controls had increased sleep during the dark period and displayed increased limb movements during REM sleep. None of the lesioned rats had sleep onset REM sleep periods or cataplexy. We conclude that the hypocretin innervation to the pons functions to awaken the animal when the lights turn off (via its innervation of the LC), sustains arousal and represses sleep during the rest of the night (via a wider innervation of other pontine neurons), and modulates muscle tone.
Figures







References
-
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nature Neurosci. 2001;4:732–738. - PubMed
-
- Blessing WW, Lappi DA, Wiley RG. Destruction of locus coeruleus neuronal perikarya after injection of anti-dopamine-B-hydroxylase immunotoxin into the olfactory bulb of the rat. Neurosci Lett. 1998;243:85–88. - PubMed
-
- Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. Neuroreport. 1995;6:532–536. - PubMed
-
- Bremer F. Isolated brain and physiology of sleep. CR Soc Biol. 1935;118:1235–1241.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

