Induction of hyperthyroidism in mice by intradermal immunization with DNA encoding the thyrotropin receptor
- PMID: 15147342
- PMCID: PMC1809053
- DOI: 10.1111/j.1365-2249.2004.02483.x
Induction of hyperthyroidism in mice by intradermal immunization with DNA encoding the thyrotropin receptor
Abstract
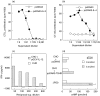
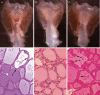
Intramuscular injection with plasmid DNA encoding the human thyrotropin receptor (TSHR) has been known to elicit symptoms of Graves' disease (GD) in outbred but not inbred mice. In this study, we have examined, firstly, whether intradermal (i.d.) injection of TSHR DNA can induce hyperthyroidism in BALB/c mice and, secondly, whether coinjection of TSHR- and cytokine-producing plasmids can influence the outcome of disease. Animals were i.d. challenged at 0, 3 and 6 weeks with TSHR DNA and the immune response was assessed at the end of the 8th or 10th week. In two experiments, a total of 10 (67%) of 15 mice developed TSHR-specific antibodies as assessed by flow cytometry. Of these, 4 (27%) mice had elevated thyroxine (TT4) levels and goitrous thyroids with activated follicular epithelial cells but no evidence of lymphocytic infiltration. At 10 weeks, thyroid-stimulating antibodies (TSAb) were detected in two out of the four hyperthyroid animals. Interestingly, in mice that received a coinjection of TSHR- and IL-2- or IL-4-producing plasmids, there was no production of TSAbs and no evidence of hyperthyroidism. On the other hand, coinjection of DNA plasmids encoding TSHR and IL-12 did not significantly enhance GD development since two out of seven animals became thyrotoxic, but had no goitre. These results demonstrate that i.d. delivery of human TSHR DNA can break tolerance and elicit GD in inbred mice. The data do not support the notion that TSAb production is Th2-dependent in murine GD but they also suggest that codelivery of TSHR and Th1-promoting IL-12 genes may not be sufficient to enhance disease incidence and/or severity in this model.
Figures

Similar articles
-
TSH receptor-adenovirus-induced Graves' hyperthyroidism is attenuated in both interferon-gamma and interleukin-4 knockout mice; implications for the Th1/Th2 paradigm.Clin Exp Immunol. 2004 Dec;138(3):417-22. doi: 10.1111/j.1365-2249.2004.02641.x. Clin Exp Immunol. 2004. PMID: 15544617 Free PMC article.
-
Adenovirus encoding the thyrotropin receptor A-subunit improves the efficacy of dendritic cell-induced Graves' hyperthyroidism in mice.J Autoimmun. 2006 Feb;26(1):32-6. doi: 10.1016/j.jaut.2005.08.008. Epub 2005 Oct 18. J Autoimmun. 2006. PMID: 16242303
-
Induction of experimental autoimmune Graves' disease in BALB/c mice.J Immunol. 1999 Nov 1;163(9):5157-64. J Immunol. 1999. PMID: 10528222
-
Insight into Graves' hyperthyroidism from animal models.Endocr Rev. 2005 Oct;26(6):800-32. doi: 10.1210/er.2004-0023. Epub 2005 Apr 12. Endocr Rev. 2005. PMID: 15827111 Review.
-
[Progress in the pathogenesis of Graves' disease: approaches from animal models].Nihon Rinsho. 2006 Dec;64(12):2215-8. Nihon Rinsho. 2006. PMID: 17154081 Review. Japanese.
Cited by
-
Insight Into Mouse Models of Hyperthyroidism.Front Endocrinol (Lausanne). 2022 Jun 22;13:929750. doi: 10.3389/fendo.2022.929750. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35813642 Free PMC article. Review.
-
Effects of hyperthyroidism on the rectus muscles in mice.Front Neurol. 2010 Nov 11;1:143. doi: 10.3389/fneur.2010.00143. eCollection 2010. Front Neurol. 2010. PMID: 21212842 Free PMC article.
-
Excessive Cytosolic DNA Fragments as a Potential Trigger of Graves' Disease: An Encrypted Message Sent by Animal Models.Front Endocrinol (Lausanne). 2016 Nov 14;7:144. doi: 10.3389/fendo.2016.00144. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27895620 Free PMC article. Review.
-
TSH receptor-adenovirus-induced Graves' hyperthyroidism is attenuated in both interferon-gamma and interleukin-4 knockout mice; implications for the Th1/Th2 paradigm.Clin Exp Immunol. 2004 Dec;138(3):417-22. doi: 10.1111/j.1365-2249.2004.02641.x. Clin Exp Immunol. 2004. PMID: 15544617 Free PMC article.
-
Adenovirus-mediated gene delivery of interleukin-10, but not transforming growth factor beta, ameliorates the induction of Graves' hyperthyroidism in BALB/c mice.Clin Exp Immunol. 2005 Sep;141(3):405-11. doi: 10.1111/j.1365-2249.2005.02874.x. Clin Exp Immunol. 2005. PMID: 16045729 Free PMC article.
References
-
- Rapoport B. The thyrotropin receptor. In: Braverman LE, Utiger RD, editors. Werner & Ingbar's The Thyroid: a fundamental and clinical text. Philadelphia: Lippincott, Williams & Wilkins; 2000. pp. 219–27.
-
- Davies TF. Graves’ Disease. Pathogenesis. In: Braverman LE, Utiger RD, editors. Werner & Ingbar's The Thyroid: a fundamental and clinical text. Philadelphia: Lippincott, Williams & Wilkins; 2000. pp. 518–31.
-
- Ludgate M. Animal models of Graves’ disease. Eur J Endocrinol. 2000;142:1–8. - PubMed
-
- Marion S, Braun JM, Ropars A, Kohn LD, Charreire J. Induction of autoimmunity by immunization of mice with human thyrotropin receptor. Cell Immunol. 1994;158:329–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

