The roles of interleukin-18 in collagen-induced arthritis in the BB rat
- PMID: 15147345
- PMCID: PMC1809057
- DOI: 10.1111/j.1365-2249.2004.02430.x
The roles of interleukin-18 in collagen-induced arthritis in the BB rat
Abstract
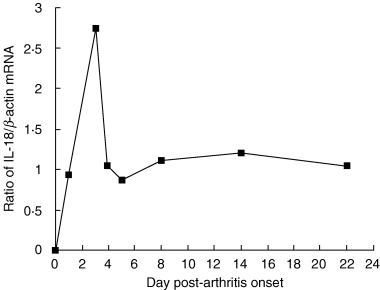
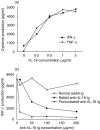
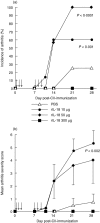
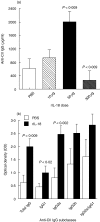
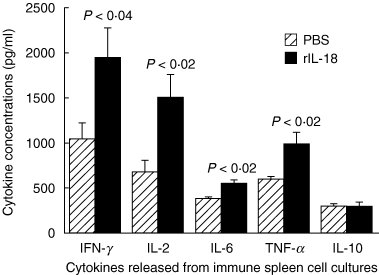
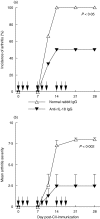
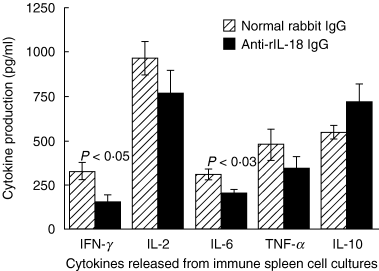
Interleukin (IL)-18 is a member of the IL-1 cytokine family. Its expression is increased in rheumatoid arthritis synovium, and its proinflammatory effects have been demonstrated in experimental models of murine arthritis. Here, we investigate the actions of varying doses of recombinant rat IL-18 (rIL-18) on the course of type II collagen-induced arthritis (CIA) in BB rats, including clinical and immune events, plus splenic cytokine production. Small doses of rIL-18 (10 and 50 microg/rat) administered intraperitoneally (i.p.) increased arthritis incidence and severity (P < 0.01) when a low-potency CII preparation was used for immunization. IgG1 and IgG2a anti-CII antibody levels were significantly greater in rats given 10 and 50 microg rIL-18 doses than controls. rIL-18 significantly increased levels of proinflammatory cytokines [interferon (IFN)-gamma, IL-2, tumour necrosis factor (TNF)-alpha and IL-6] produced by splenocyte cultures. Larger doses of rIL-18 (300 microg/rat) suppressed arthritis and immunity. To ascertain whether the pro-arthritic effects of IL-18 could be attenuated, rats were treated with neutralizing rabbit anti-rIL-18 IgG before immunization with a high-potency CII preparation. When given serially for 3 weeks, the incidence and severity of CIA, in addition to anti-CII IgG2a and splenic IL-6 and IFN-gamma production, were all significantly reduced. Similar results were noted when antibody was given twice, just before arthritis onset. These results demonstrate that IL-18 plays an important proinflammatory role in the pathogenesis of CIA which is achieved, in part, by an immunostimulatory action. Neutralizing endogenous IL-18 with antibodies attenuated CIA, CII immunity and cytokine responses. These studies support the use of IL-18 antagonists as treatments for inflammatory arthritis.
Figures
Similar articles
-
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article.
-
Torilin ameliorates type II collagen-induced arthritis in mouse model of rheumatoid arthritis.Int Immunopharmacol. 2013 Jun;16(2):232-42. doi: 10.1016/j.intimp.2013.04.012. Epub 2013 Apr 24. Int Immunopharmacol. 2013. PMID: 23623942
-
Kirenol exerts a potent anti-arthritic effect in collagen-induced arthritis by modifying the T cells balance.Phytomedicine. 2012 Jul 15;19(10):882-9. doi: 10.1016/j.phymed.2012.04.010. Epub 2012 Jun 4. Phytomedicine. 2012. PMID: 22673798
-
Protective effects of Huang-Lian-Jie-Du-Tang and its component group on collagen-induced arthritis in rats.J Ethnopharmacol. 2013 Dec 12;150(3):1137-44. doi: 10.1016/j.jep.2013.10.038. Epub 2013 Nov 7. J Ethnopharmacol. 2013. PMID: 24212076
-
Pro- and anti-inflammatory cytokines in rheumatoid arthritis.Ann Med. 1997 Dec;29(6):499-507. doi: 10.3109/07853899709007474. Ann Med. 1997. PMID: 9562516 Review.
Cited by
-
Therapeutic effect of low-dose IL-18 combined with IL-10 on collagen-induced arthritis by down-regulation of inflammatory and Th1 responses and induction of Th2 responses.Rheumatol Int. 2009 Apr;29(6):615-22. doi: 10.1007/s00296-008-0732-3. Epub 2008 Oct 9. Rheumatol Int. 2009. PMID: 18841371
-
Cellular targets of interleukin-18 in rheumatoid arthritis.Ann Rheum Dis. 2007 Nov;66(11):1411-8. doi: 10.1136/ard.2006.067793. Epub 2007 May 14. Ann Rheum Dis. 2007. PMID: 17502360 Free PMC article. Review.
-
Changes in microarchitectural characteristics at the tibial epiphysis induced by collagen-induced rheumatoid arthritis over time.Clin Interv Aging. 2012;7:373-82. doi: 10.2147/CIA.S35202. Epub 2012 Sep 18. Clin Interv Aging. 2012. PMID: 23049249 Free PMC article.
-
Interleukin-18 as a potential target in inflammatory arthritis.Clin Exp Immunol. 2004 Jun;136(3):402-4. doi: 10.1111/j.1365-2249.2004.02475.x. Clin Exp Immunol. 2004. PMID: 15147340 Free PMC article. Review. No abstract available.
-
Expression of interleukin-18, IL-18BP, and IL-18R in serum, synovial fluid, and synovial tissue in patients with rheumatoid arthritis.Clin Exp Med. 2009 Sep;9(3):215-21. doi: 10.1007/s10238-009-0036-2. Epub 2009 Feb 19. Clin Exp Med. 2009. PMID: 19225717
References
-
- Griffiths MM, Cremer MA, Harper DS, McCall S, Cannon GW. Immunogenetics of collagen-induced arthritis in rats. Both MHC and non-MHC gene products determine the epitope specificity of immune response to bovine and chick type II collagens. J Immunol. 1992;149:309–16. - PubMed
-
- Joosten LA, Helsen MM, van de Loo FA, van den Berg WB. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

