Recognition of nonpeptide antigens by human V gamma 9V delta 2 T cells requires contact with cells of human origin
- PMID: 15147349
- PMCID: PMC1809052
- DOI: 10.1111/j.1365-2249.2004.02472.x
Recognition of nonpeptide antigens by human V gamma 9V delta 2 T cells requires contact with cells of human origin
Abstract
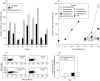
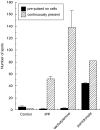
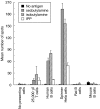
SUMMARY It is becoming apparent that gamma delta T cells form an important part of the adaptive immune response. However, the ligands recognized by gamma delta T cell receptors (TCRs) and the exact biological function of the cells that express this receptor remain unclear. Numerous studies have shown that the dominant human peripheral blood subset of gamma delta T cells, which express a V gamma 9V delta 2 TCR, can activate in response to low molecular weight nonpeptidic molecules. Some of these components have been purified from bacteria or parasites. We examined the activation of polyclonal gamma delta T cell lines, clones with V gamma 9V delta 2 and V gamma 9V delta 1 TCRs, and gamma delta T cells directly ex vivo in response to multiple phosphate, alkylamine and aminobisphosphonate (nBP) antigens and purified protein derivative from Mycobacterium tuberculosis (PPD). V gamma 9V delta 2 T cells were able to respond to multiple small organic molecules of highly variable structure whereas cells expressing a similar V gamma 9 chain paired with a V delta 1 chain failed to recognize these antigens. Thus, the TCR delta chain appears to make an important contribution to the recognition of these antigens. The kinetics of responses to alkylphosphate and alkylamine antigens differ from those of responses to the nBP pamidronate. These different classes of antigen are believed to have differed mechanisms of action. Such differences explain why nBPs can be pulsed onto antigen presenting cells (APCs) and still retain their ability to activate gamma delta T cells while alkylphosphate and alkylamine antigens cannot. We also demonstrate that a substantial proportion of the cells that produce IFN gamma directly ex vivo in response to PPD are gamma delta T cells and that gamma delta T cell activation requires contact with cells of human origin.
Figures



References
-
- Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. - PubMed
-
- Sciammas R, Tatsumi Y, Sperling AI, Arunan K, Bluestone JA. TCR gamma delta cells: mysterious cells of the immune system. Immunol Res. 1994;13:268–79. - PubMed
-
- Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. - PubMed
-
- Balbi B, Valle MT, Oddera S, Giunti D, Manca F, Rossi GA, Allegra L. T-lymphocytes with gamma delta+ V delta 2+ antigen receptors are present in increased proportions in a fraction of patients with tuberculosis or with sarcoidosis. Am Rev Respir Dis. 1993;148:1685–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

