Structural and functional complexity of the humoral response against the Trypanosoma cruzi ribosomal P2 beta protein in patients with chronic Chagas' heart disease
- PMID: 15147356
- PMCID: PMC1809055
- DOI: 10.1111/j.1365-2249.2004.02480.x
Structural and functional complexity of the humoral response against the Trypanosoma cruzi ribosomal P2 beta protein in patients with chronic Chagas' heart disease
Abstract
High levels of antibodies against the C-terminus of the Trypanosoma cruzi TcP2 beta ribosomal protein, defined by the peptide EEEDDDMGFGLFD, named R13, have been measured in sera from patients with chronic Chagas' Heart Disease (cChHD). These antibodies also recognize an epitope on the second extracellular loop of the beta 1-adrenergic receptor, inducing a functional response on cardiomyocytes. The aim of this study was to gain novel insights into the structural basis of this cross-reactivity as well as to evaluate the origin of anti-M2- cholinergic receptor antibodies, which are also commonly found in cChHD patients. To address these questions we immunopurified anti-R13 antibodies and studied the structural requirements of epitope recognition. Results showed that the immunopurified antibodies recognized a conformation of R13 in which the third Glu residue was essential for binding, explaining their low affinity for the mammalian homologue (peptide H13: EESDDDMGFGLFD). Alanine mutation scanning showed individual variations in epitope recognition in each of the studied patients. The importance of a negatively charged residue at position 3 for the recognition of anti-R13 antibodies was further confirmed by competition experiments using a Ser3-phosphorylated H13 analogue, which had 10 times more affinity for the anti-R13 antibody than the native H13 peptide. Moreover, anti-R13 antibodies stimulated either the beta 1-adrenergic or the M2-cholinergic receptor, in strict agreement with the functional properties of the IgG fractions from which they derived, demonstrating that the same parasite antigen may generate antibody specificities with different functional properties. This may be a clue to explain the high variability of electrophysiological disturbances found in cChHD.
Figures
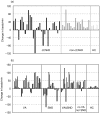
 ) and healthy individuals (□). Bars represent change in beating rate caused by addition of IgG fraction from each individual. All results represent one from at least three independent experiments. (b) Chronotropic effect of IgG fractions (▪) among the cChHD population. The specificity of the effect was studied by subsequent addition of the M2-ChR antagonist atropine (□) and the β1-AR antagonist bisoprolol (
) and healthy individuals (□). Bars represent change in beating rate caused by addition of IgG fraction from each individual. All results represent one from at least three independent experiments. (b) Chronotropic effect of IgG fractions (▪) among the cChHD population. The specificity of the effect was studied by subsequent addition of the M2-ChR antagonist atropine (□) and the β1-AR antagonist bisoprolol ( ).VA, Ventricular arrhythmias; SND, Sinus node dysfunction; HC, Healthy Controls.
).VA, Ventricular arrhythmias; SND, Sinus node dysfunction; HC, Healthy Controls.
 ), and CH group IV (n = 3): preclinical chronic Chagas cardiomyopathy with no ECG disturbances (e). Error bars represent standard deviation. Significance of the differences in mean antibody levels between CH group I and CH groups III + IV were determined applying the Wilcoxon test (P < 0·05).
), and CH group IV (n = 3): preclinical chronic Chagas cardiomyopathy with no ECG disturbances (e). Error bars represent standard deviation. Significance of the differences in mean antibody levels between CH group I and CH groups III + IV were determined applying the Wilcoxon test (P < 0·05).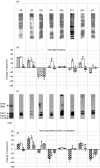
 ), as well as antagonizing effects of 10 µ
), as well as antagonizing effects of 10 µ ) were evaluated. (c) Immunoblotting of T. cruzi epimastigote lysate with the eight purified anti-R13 antibodies. The monoclonal antibody mAb17·2 was used as positive control. Arrows indicate the position of the T. cruzi ribosomal P0 protein and the low molecular weight ribosomal P proteins (TcP1, TcP2α and TcP2β) (d) Chronotropic effect of the corresponding immunopurified purified anti-R13 antibodies (
) were evaluated. (c) Immunoblotting of T. cruzi epimastigote lysate with the eight purified anti-R13 antibodies. The monoclonal antibody mAb17·2 was used as positive control. Arrows indicate the position of the T. cruzi ribosomal P0 protein and the low molecular weight ribosomal P proteins (TcP1, TcP2α and TcP2β) (d) Chronotropic effect of the corresponding immunopurified purified anti-R13 antibodies ( ). Bars represent mean ± SD of the change in beats/min; *significant differences (P < 0·001) with respect to the preceding bar.
). Bars represent mean ± SD of the change in beats/min; *significant differences (P < 0·001) with respect to the preceding bar.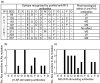
Similar articles
-
A monoclonal antibody against the immunodominant epitope of the ribosomal P2beta protein of Trypanosoma cruzi interacts with the human beta 1-adrenergic receptor.Eur J Immunol. 2001 Jul;31(7):2210-6. doi: 10.1002/1521-4141(200107)31:7<2210::aid-immu2210>3.0.co;2-j. Eur J Immunol. 2001. PMID: 11449375
-
The beta1 adrenergic effects of antibodies against the C-terminal end of the ribosomal P2beta protein of Trypanosoma cruzi associate with a specific pattern of epitope recognition.Clin Exp Immunol. 2005 Oct;142(1):140-7. doi: 10.1111/j.1365-2249.2005.02885.x. Clin Exp Immunol. 2005. PMID: 16178868 Free PMC article.
-
Antibodies to ribosomal P proteins of Trypanosoma cruzi in Chagas disease possess functional autoreactivity with heart tissue and differ from anti-P autoantibodies in lupus.Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10301-6. doi: 10.1073/pnas.94.19.10301. Proc Natl Acad Sci U S A. 1997. PMID: 9294205 Free PMC article.
-
Humoral autoimmune response in Chagas' disease: Trypanosoma cruzi ribosomal antigens as immunizing agents.FEMS Immunol Med Microbiol. 1993 Oct;7(3):205-10. doi: 10.1111/j.1574-695X.1993.tb00400.x. FEMS Immunol Med Microbiol. 1993. PMID: 7506093 Review.
-
Role of autoantibodies in the physiopathology of Chagas' disease.Arq Bras Cardiol. 2008 Oct;91(4):257-62, 281-6. doi: 10.1590/s0066-782x2008001600012. Arq Bras Cardiol. 2008. PMID: 19009179 Free PMC article. Review.
Cited by
-
Anti-beta1-adrenergic receptor autoantibodies in patients with chronic Chagas heart disease.Clin Exp Immunol. 2007 Jun;148(3):440-9. doi: 10.1111/j.1365-2249.2007.03381.x. Epub 2007 Apr 5. Clin Exp Immunol. 2007. PMID: 17419712 Free PMC article.
-
Cytokine production but lack of proliferation in peripheral blood mononuclear cells from chronic Chagas' disease cardiomyopathy patients in response to T. cruzi ribosomal P proteins.PLoS Negl Trop Dis. 2014 Jun 5;8(6):e2906. doi: 10.1371/journal.pntd.0002906. eCollection 2014 Jun. PLoS Negl Trop Dis. 2014. PMID: 24901991 Free PMC article.
-
Evaluation of in-house ELISA using Trypanosoma cruzi lysate and recombinant antigens for diagnosis of Chagas disease and discrimination of its clinical forms.Am J Trop Med Hyg. 2012 Aug;87(2):267-71. doi: 10.4269/ajtmh.2012.11-0533. Am J Trop Med Hyg. 2012. PMID: 22855757 Free PMC article.
-
Crystal structure of the complex mAb 17.2 and the C-terminal region of Trypanosoma cruzi P2β protein: implications in cross-reactivity.PLoS Negl Trop Dis. 2011 Nov;5(11):e1375. doi: 10.1371/journal.pntd.0001375. Epub 2011 Nov 1. PLoS Negl Trop Dis. 2011. PMID: 22069505 Free PMC article.
-
The diagnostic and clinical significance of anti-muscarinic receptor autoantibodies.Clin Rev Allergy Immunol. 2012 Jun;42(3):298-308. doi: 10.1007/s12016-010-8235-x. Clin Rev Allergy Immunol. 2012. PMID: 21207192 Review.
References
-
- Elizari MV, Chiale PA. Cardiac arrhythmias in Chagas’ heart disease. J Cardiovasc Electrophysiol. 1993;4:596–608. - PubMed
-
- Rassi A, Lorga AM, Rassi SG. Diagnostic and therapeutic approach of the chronic Chagas-related cardiopathy. In: Germiniani H, editor. Diagnosis and Treatment of Cardiac Arrhythmias. 3. Rio de Janeiro: Guanabara Koogan S.A.; 1990. pp. 225–44.
-
- Halperin C, Rassi S. Clinical Relevance of Invasive Electrophysiologic Studies in Patients with Chagas’ Disease. In: Armonk NY, editor. Arrhythmia Management in Chagas’ Disease. Futura Publishing Co Inc.; 2000. pp. 83–93.
-
- Rosenbaum MB, Chiale PA, Schejtman D, Levin M, Elizari MV. Antibodies to beta-adrenergic receptors disclosing agonist-like properties in idiopathic dilated cardiomyopathy and Chagas’ heart disease. J Cardiovasc Electrophysiol. 1994;5:367–75. - PubMed
-
- Goin JC, Borda E, Leiros CP, Storino R, Sterin-Borda L. Identification of antibodies with muscarinic cholinergic activity in human Chagas’ disease: pathological implications. J Auton Nerv Syst. 1994;47:45–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

