Impaired chemokine-induced migration during T-cell development in the absence of Jak 3
- PMID: 15147562
- PMCID: PMC1782482
- DOI: 10.1111/j.1365-2567.2004.01863.x
Impaired chemokine-induced migration during T-cell development in the absence of Jak 3
Abstract
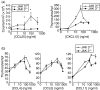
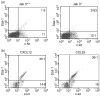
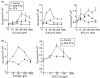
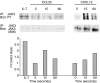
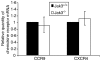
The arrival of bone marrow T-cell progenitors to the thymus, and the directed migration of thymocytes, are thought to be regulated by the expression of chemokines and their receptors. Recent data has shown that the Jak/Stat signalling pathway is involved in chemokine receptor signalling. We have investigated the role of Jak 3 in chemokine-mediated signalling in the thymus using Jak 3(-/-) mice. These mice show defects in T-cell development, as well as in peripheral T-cell function, resulting in a hypoplastic thymus and an altered T-cell homeostasis. Here we demonstrate, for the first time, that bone marrow progenitors and thymocytes from Jak 3(-/-) mice have decreased chemotactic responses to CXCL12 and CCL25. We also show that Jak 3 is involved in signalling through CCR9 and CXCR4, and that specific inhibition of Jak 3 in wild-type progenitors and thymocytes decreases their chemotactic responses towards CCL25 and CXCL12. Finally, quantitative reverse transcription-polymerase chain reaction analysis showed that thymocytes from Jak 3(-/-) mice express similar levels of CXCR4 and CCR9 compared to wild-type mice. Altogether, deficient CCL25- and CXCL12-induced migration could result in a homing defect of T-cell progenitors to the thymus, as well as in a deficient thymocyte migration through the thymic stroma. Our results strongly suggest that the absence of Jak 3 affects T-cell development, not only through an impaired interleukin-7 receptor (IL-7R)-mediated signalling, but also through impaired chemokine-mediated responses, which are crucial for thymocyte migration and differentiation.
Figures
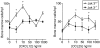
Similar articles
-
Aberrant T-cell ontogeny and defective thymocyte and colonic T-cell chemotactic migration in colitis-prone Galphai2-deficient mice.Immunology. 2007 Oct;122(2):199-209. doi: 10.1111/j.1365-2567.2007.02629.x. Epub 2007 May 9. Immunology. 2007. PMID: 17490434 Free PMC article.
-
Janus kinase 3-deficient T lymphocytes have an intrinsic defect in CCR7-mediated homing to peripheral lymphoid organs.Immunology. 2007 Oct;122(2):247-60. doi: 10.1111/j.1365-2567.2007.02634.x. Epub 2007 May 22. Immunology. 2007. PMID: 17521370 Free PMC article.
-
GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo.J Leukoc Biol. 2004 Apr;75(4):698-704. doi: 10.1189/jlb.0703320. Epub 2004 Jan 2. J Leukoc Biol. 2004. PMID: 14704365
-
Roles of chemokines in thymopoiesis: redundancy and regulation.Cell Mol Immunol. 2004 Aug;1(4):266-73. Cell Mol Immunol. 2004. PMID: 16225769 Review.
-
Molecular mechanisms governing thymocyte migration: combined role of chemokines and extracellular matrix.J Leukoc Biol. 2004 Jun;75(6):951-61. doi: 10.1189/jlb.1003455. Epub 2004 Mar 12. J Leukoc Biol. 2004. PMID: 15020651 Review.
Cited by
-
Immunogenic profiling in mice of a HIV/AIDS vaccine candidate (MVA-B) expressing four HIV-1 antigens and potentiation by specific gene deletions.PLoS One. 2010 Aug 24;5(8):e12395. doi: 10.1371/journal.pone.0012395. PLoS One. 2010. PMID: 20811493 Free PMC article.
-
CXCR4 in breast cancer: oncogenic role and therapeutic targeting.Drug Des Devel Ther. 2015 Aug 28;9:4953-64. doi: 10.2147/DDDT.S84932. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26356032 Free PMC article. Review.
-
Immunohistological analysis of the jun family and the signal transducers and activators of transcription in thymus.Anat Res Int. 2015;2015:541582. doi: 10.1155/2015/541582. Epub 2015 Mar 18. Anat Res Int. 2015. PMID: 25866678 Free PMC article.
-
Bidirectional Regulation of Opioid and Chemokine Function.Front Immunol. 2020 Jan 31;11:94. doi: 10.3389/fimmu.2020.00094. eCollection 2020. Front Immunol. 2020. PMID: 32076421 Free PMC article. Review.
-
Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology.Prog Neurobiol. 2008 Feb;84(2):116-31. doi: 10.1016/j.pneurobio.2007.11.003. Epub 2007 Nov 26. Prog Neurobiol. 2008. PMID: 18177992 Free PMC article. Review.
References
-
- Chan S, Correia-Neves M, Benoist C, Mathis D. CD4/CD8 lineage commitment: matching fate with competence. Immunol Rev. 1998;165:195–207. - PubMed
-
- Sebzda E, Mariathasan S, Ohteki T, Jones R, Bachmann MF, Ohashi PS. Selection of the T cell repertoire. Annu Rev Immunol. 1999;17:829–74. - PubMed
-
- von Boehmer H, Aifantis I, Feinberg J, Lechner O, Saint-Ruf C, Walter U, Buer J, Azogui O, et al. Pleiotropic changes controlled by the pre-T-cell receptor. Curr Opin Immunol. 1999;11:135–42. - PubMed
-
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. - PubMed
-
- Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1:31–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
