Selective killing of B-cell hybridomas targeting proteinase 3, Wegener's autoantigen
- PMID: 15147566
- PMCID: PMC1782481
- DOI: 10.1111/j.1365-2567.2004.01875.x
Selective killing of B-cell hybridomas targeting proteinase 3, Wegener's autoantigen
Abstract
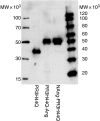
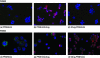
Wegener's granulomatosis (WG) is a rare disease characterized by granulomatous lesions, small vessel vasculitis and the presence of anti-neutrophil cytoplasmic autoantibodies (C-ANCAs) in the sera of affected patients. Their main target antigen is proteinase 3 (PR3), a neutrophil and monocyte-derived neutral serine protease. Since the standard treatment of this severe autoimmune disease, with cyclophosphamide and corticosteroids, is associated with potential side-effects, the development of a more specific immunotherapeutic agent is warranted. The key role of ANCA in the pathogenesis of vasculitis and the effectiveness of anti-CD20 antibodies in patients with refractory WG points towards the importance of B cells in WG. We thus evaluated a new approach to selectively eliminate PR3-specific autoreactive B cells by targeting the B-cell receptor. For this purpose we used a bifunctional recombinant fusion protein consisting of the antigen PR3 and a toxin. The cytotoxic component of this novel fusion protein was the ribonuclease angiogenin, a human toxin with low immunogenicity. The toxin was stabilized by exchanging the catalytically relevant histidine in position 44 with glutamine to eliminate the autoproteolytic activity. PR3H44Q was fused either to the N terminus or to the C terminus of angiogenin. The recombinant proteins were expressed in 293T cells. Binding assays demonstrated the appropriate size and recognition by anti-PR3 antibodies. Using TUNEL technology, we demonstrated that these autoantigen toxins kill proteinase 3-specific B-cell hybridomas selectively by inducing apoptosis. The data indicate that autoantigen-toxins are promising tools in the treatment or co-treatment of autoimmune diseases in which the antigen is known.
Figures

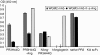
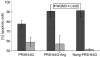
Similar articles
-
'Classic' anti-neutrophil cytoplasmic autoantibodies (cANCA), 'Wegener's autoantigen' and their immunopathogenic role in Wegener's granulomatosis.J Autoimmun. 1993 Apr;6(2):171-84. doi: 10.1006/jaut.1993.1015. J Autoimmun. 1993. PMID: 8388690 Review.
-
Proteinase 3, Wegener's autoantigen: from gene to antigen.J Leukoc Biol. 2001 Feb;69(2):177-90. J Leukoc Biol. 2001. PMID: 11272267 Review.
-
Is PR3-ANCA formation initiated in Wegener's granulomatosis lesions? Granulomas as potential lymphoid tissue maintaining autoantibody production.Ann N Y Acad Sci. 2005 Jun;1051:12-9. doi: 10.1196/annals.1361.042. Ann N Y Acad Sci. 2005. PMID: 16126940 Review.
-
Expression of the human autoantigen of Wegener's granulomatosis (PR3) in a baculovirus expression system.Biochem Biophys Res Commun. 1996 Feb 15;219(2):283-9. doi: 10.1006/bbrc.1996.0224. Biochem Biophys Res Commun. 1996. PMID: 8604978
-
Antineutrophil cytoplasmic autoantibodies in Wegener's granulomatosis recognize conformational epitope(s) on proteinase 3.J Immunol. 1992 Aug 15;149(4):1409-15. J Immunol. 1992. PMID: 1380042
Cited by
-
Effect of Antigen Valency on Autoreactive B-Cell Targeting.Mol Pharm. 2024 Feb 5;21(2):481-490. doi: 10.1021/acs.molpharmaceut.3c00527. Epub 2023 Oct 20. Mol Pharm. 2024. PMID: 37862070 Free PMC article.
-
Engineered human angiogenin mutations in the placental ribonuclease inhibitor complex for anticancer therapy: Insights from enhanced sampling simulations.Protein Sci. 2016 Aug;25(8):1451-60. doi: 10.1002/pro.2941. Epub 2016 May 19. Protein Sci. 2016. PMID: 27110669 Free PMC article.
-
Sequential Prodrug Strategy To Target and Eliminate ACPA-Selective Autoreactive B Cells.Mol Pharm. 2018 Dec 3;15(12):5565-5573. doi: 10.1021/acs.molpharmaceut.8b00741. Epub 2018 Oct 26. Mol Pharm. 2018. PMID: 30289723 Free PMC article.
References
-
- Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–50. - PubMed
-
- Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, Rottem M, Fauci AS. Wegener granulomatosis. An analysis of 158 patients. Ann Intern Med. 1992;116:488–98. - PubMed
-
- Stillwell TJ, Benson RC, Jr, DeRemee RA, McDonald TJ, Weiland LH. Cyclophosphamide-induced bladder toxicity in Wegener's granulomatosis. Arthritis Rheum. 1988;31:465–70. - PubMed
-
- Radis CD, Kahl LE, Baker GL, et al. Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study. Arthritis Rheum. 1995;38:1120–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
