Aberration of CCR7 CD8 memory T cells from patients with systemic lupus erythematosus: an inducer of T helper type 2 bias of CD4 T cells
- PMID: 15147571
- PMCID: PMC1782491
- DOI: 10.1111/j.1365-2567.2004.01862.x
Aberration of CCR7 CD8 memory T cells from patients with systemic lupus erythematosus: an inducer of T helper type 2 bias of CD4 T cells
Abstract
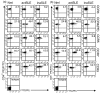
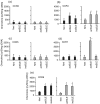
Chemokine receptors are important in the entry of leucocytes into the inflammatory sites of systemic lupus erythematosus (SLE). CCR7(+) and CCR7(-) memory T cells exert different functions in homing, cytokine production and cytotoxicity. To determine whether differential expression and functions of the CCR7 occur in SLE patients, we examined CCR3, CCR4, CCR5, CCR7 and CCR9 on CD4(+) and CD8(+) T cells from normal and SLE subjects. Flow cytometry, real-time quantitative reverse transcription polymerase chain reactions and Northern blotting were used to detect the expression of chemokine receptors and cytokines; a chemotaxis assay was used to detect their functions. CD4(+) T-cell stimulation with syngeneic CCR7(+) CD8(+) CD45RO(+) T cells and dendritic cells (including transwell chambers) was used to induce cytokine expression. We demonstrated that CCR7 was selectively, frequently and functionally expressed on CD8(+) (94.8%) but not on CD4(+) (16.1%) T cells from patients with active SLE, whereas this phenomenon was not seen in normal subjects and in those whose SLE was inactive. CCR7(+) CD8(+) CD45RO(+) memory T cells from patients with active SLE, themselves T helper type 2 (Th2) biased, were inducers of Th2 bias in CD4(+) T cells in a cell-cell contact manner in vitro, meanwhile, the cells from both normal subjects and those whose SLE was inactive drove CD4(+) T cells into a regulatory T-cell-derived cytokine pattern. Our findings might provide new clues to understanding the functions of CCR7(+) CD8(+) CD45RO(+)'central' memory T cells in autoimmune diseases (such as SLE). We suggest that in the case of active SLE, CCR7(+) central memory T cells were able to enter peripheral blood and inflammatory sites from secondary lymphoid organs, were continuously expressing CCR7, and interacted with dendritic cells and functioned as CCR7(-)'effector' memory T cells, which were described in normal humans.
Figures








Similar articles
-
Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells.J Immunol. 2002 Mar 1;168(5):2225-32. doi: 10.4049/jimmunol.168.5.2225. J Immunol. 2002. PMID: 11859109
-
Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype.J Immunol. 2002 Jun 1;168(11):5538-50. doi: 10.4049/jimmunol.168.11.5538. J Immunol. 2002. PMID: 12023349
-
Persistent expansion of CD4+ effector memory T cells in Wegener's granulomatosis.Kidney Int. 2006 Sep;70(5):938-47. doi: 10.1038/sj.ki.5001670. Epub 2006 Jul 12. Kidney Int. 2006. PMID: 16837919
-
Inhibitory CD8+ T cells in autoimmune disease.Hum Immunol. 2008 Nov;69(11):781-9. doi: 10.1016/j.humimm.2008.08.283. Epub 2008 Sep 21. Hum Immunol. 2008. PMID: 18812196 Free PMC article. Review.
-
Induction of immune tolerance by activation of CD8+ T suppressor/regulatory cells in lupus-prone mice.Hum Immunol. 2008 Nov;69(11):790-6. doi: 10.1016/j.humimm.2008.08.284. Epub 2008 Sep 24. Hum Immunol. 2008. PMID: 18817829 Free PMC article. Review.
Cited by
-
Peripheral blood T-cell subset and its clinical significance in lupus nephritis patients.Lupus Sci Med. 2022 Aug;9(1):e000717. doi: 10.1136/lupus-2022-000717. Lupus Sci Med. 2022. PMID: 35973743 Free PMC article.
-
Different Types of Chronic Inflammation Engender Distinctive Immunosenescent Profiles in Affected Patients.Int J Mol Sci. 2022 Nov 24;23(23):14688. doi: 10.3390/ijms232314688. Int J Mol Sci. 2022. PMID: 36499016 Free PMC article.
-
Disease Stage-Specific Pathogenicity of CD138 (Syndecan 1)-Expressing T Cells in Systemic Lupus Erythematosus.Front Immunol. 2020 Jul 28;11:1569. doi: 10.3389/fimmu.2020.01569. eCollection 2020. Front Immunol. 2020. PMID: 32849532 Free PMC article.
-
T Cell Transcriptomes Describe Patient Subtypes in Systemic Lupus Erythematosus.PLoS One. 2015 Nov 6;10(11):e0141171. doi: 10.1371/journal.pone.0141171. eCollection 2015. PLoS One. 2015. PMID: 26544975 Free PMC article.
-
CD8+ T Cell Phenotype and Function in Childhood and Adult-Onset Connective Tissue Disease.Int J Mol Sci. 2022 Sep 28;23(19):11431. doi: 10.3390/ijms231911431. Int J Mol Sci. 2022. PMID: 36232733 Free PMC article. Review.
References
-
- Rollins BJ. Chemokines. Blood. 1997;90:909–28. - PubMed
-
- Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. - PubMed
-
- Yamada M, Yagita H, Inoue H, et al. Selective accumulation of CCR4+ T lymphocytes into renal tissue of patients with lupus nephritis. Arthritis Rheum. 2002;46:735–40. - PubMed
-
- Furuichi K, Wada T, Sakai N, et al. Distinct expression of CCR1 and CCR5 in glomerular and interstitial lesions of human glomerular diseases. Am J Nephrol. 2000;20:291–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
