Ectopic expression of CCL19 impairs alloimmune response in mice
- PMID: 15147573
- PMCID: PMC1782480
- DOI: 10.1111/j.1365-2567.2004.01859.x
Ectopic expression of CCL19 impairs alloimmune response in mice
Abstract
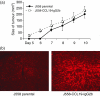
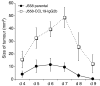
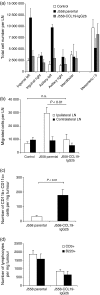
Initiation of an antitumour immune response is characterized by a complex process of chemokine-mediated cell migration and cell-cell interactions. Overexpression of chemokine CCL19 in tumour cells has been shown to result in accelerated tumour rejection under certain experimental conditions, suggesting a novel approach in the therapy of neoplastic malignancies. To investigate CCL19-mediated modulations of cellular immune responses in vivo, we generated a chimeric CCL19-immunoglobulin G2b (IgG2b) Fc fusion protein, which binds to the chemokine receptor CCR7 comparable to native CCL19. CCL19-IgG2b possesses a long-lasting potent chemotactic activity as a result of the extended half-life of Fc fusion proteins. Stable overexpression of CCL19-IgG2b in BALB/c-derived J558L tumour cells fails to support tumour cell rejection following transplantation in syngeneic mice. Moreover, overexpression of CCL19-IgG2b hinders tumour rejection in allogeneic C57BL/6 mice. This phenomenon was accompanied by a six-fold increase of dendritic cells (DCs) isolated from CCL19-IgG2b-secreting tumours when compared to the number of DCs isolated from control parental J558L tumours. While mice bearing the allogeneic parental tumour showed an intense hypercellularity in the draining lymph nodes, no such response could be observed in the draining lymph nodes of mice carrying the CCL19-IgG2b-secreting tumour. We could demonstrate that overexpression of CCL19-IgG2b in tumour cells retains antigen-presenting cells in the tumour mass and prevents DCs from migrating into draining lymph nodes to present antigens and to activate T cells, resulting in an impaired immune response against the tumour.
Figures

Similar articles
-
CCL19-IgG prevents allograft rejection by impairment of immune cell trafficking.J Am Soc Nephrol. 2006 Sep;17(9):2521-32. doi: 10.1681/ASN.2005070782. Epub 2006 Aug 9. J Am Soc Nephrol. 2006. PMID: 16899521
-
Neuroblastoma impairs chemokine-mediated dendritic cell migration in vitro.J Pediatr Surg. 2006 Jan;41(1):260-5. doi: 10.1016/j.jpedsurg.2005.10.073. J Pediatr Surg. 2006. PMID: 16410144
-
CC chemokine receptor-7 on dendritic cells is induced after interaction with apoptotic tumor cells: critical role in migration from the tumor site to draining lymph nodes.Cancer Res. 2000 Apr 15;60(8):2209-17. Cancer Res. 2000. PMID: 10786686
-
The multiple personalities of the chemokine receptor CCR7 in dendritic cells.J Immunol. 2006 May 1;176(9):5153-9. doi: 10.4049/jimmunol.176.9.5153. J Immunol. 2006. PMID: 16621978 Review.
-
Does CCL19 act as a double-edged sword in cancer development?Clin Exp Immunol. 2022 Apr 4;207(2):164-175. doi: 10.1093/cei/uxab039. Clin Exp Immunol. 2022. PMID: 35020885 Free PMC article. Review.
Cited by
-
Comparative modeling of CCRL1, a key protein in masked immune diseases and virtual screening for finding inhibitor of this protein.Bioinformation. 2012;8(7):336-40. doi: 10.6026/97320630008336. Epub 2012 Apr 13. Bioinformation. 2012. PMID: 22553392 Free PMC article.
-
Investigation of Fascin1, a Marker of Mature Dendritic Cells, Reveals a New Role for IL-6 Signaling in CCR7-Mediated Chemotaxis.J Immunol. 2021 Aug 1;207(3):938-949. doi: 10.4049/jimmunol.2000318. Epub 2021 Jul 23. J Immunol. 2021. PMID: 34301846 Free PMC article.
-
Innate signals compensate for the absence of PKC-{theta} during in vivo CD8(+) T cell effector and memory responses.Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14374-9. doi: 10.1073/pnas.0506250102. Epub 2005 Sep 26. Proc Natl Acad Sci U S A. 2005. PMID: 16186501 Free PMC article.
-
Controlled architectural and chemotactic studies of 3D cell migration.Biomaterials. 2011 Apr;32(10):2634-41. doi: 10.1016/j.biomaterials.2010.12.019. Epub 2011 Jan 14. Biomaterials. 2011. PMID: 21237507 Free PMC article.
-
Intratumoral administration of secondary lymphoid chemokine and unmethylated cytosine-phosphorothioate-guanine oligodeoxynucleotide synergistically inhibits tumor growth in vivo.J Korean Med Sci. 2011 Oct;26(10):1270-6. doi: 10.3346/jkms.2011.26.10.1270. Epub 2011 Oct 1. J Korean Med Sci. 2011. PMID: 22022177 Free PMC article.
References
-
- Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. - PubMed
-
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. - PubMed
-
- Rollins BJ. Chemokines. Blood. 1997;90:909–28. - PubMed
-
- Lipp M, Forster R, Schubel A, Burgstahler R, Muller G, Breitfeld D, Kremmer E, Wolf E. Functional organization of secondary lymphoid organs by homeostatic chemokines. Eur Cytokine Netw. 2000;11:504–5. - PubMed
-
- Yoshida R, Imai T, Hieshima K, et al. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
