Modulation of immune responses to bovine herpesvirus-1 in cattle by immunization with a DNA vaccine encoding glycoprotein D as a fusion protein with bovine CD154
- PMID: 15147576
- PMCID: PMC1782479
- DOI: 10.1111/j.1365-2567.2004.01877.x
Modulation of immune responses to bovine herpesvirus-1 in cattle by immunization with a DNA vaccine encoding glycoprotein D as a fusion protein with bovine CD154
Abstract
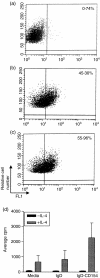
The objective of this study was to determine whether a DNA vaccine encoding bovine CD154 linked to a truncated version of bovine herpesvirus-1 (BHV-1) glycoprotein D (tgD-CD154) induces enhanced tgD-specific immune responses in cattle. In vitro characterization demonstrated that tgD and tgD-CD154 both bind to cultured bovine B cells, whereas only tgD-CD154 induces interleukin-4-dependent proliferation, suggesting that tgD-CD154 specifically binds the CD40 receptor and induces receptor signalling. Calves were immunized with plasmid encoding either tgD or tgD-CD154 by intradermal injection with a needle-free device. After two immunizations, tgD-specific immune responses were observed in both vaccinated groups and after challenge with BHV-1 these responses further increased. Animals immunized with plasmid encoding tgD-CD154 had significantly higher tgD-specific serum titres of immunoglobulins G and A but significantly lower numbers of tgD-specific interferon-gamma-secreting cells than animals immunized with plasmid encoding tgD after BHV-1 challenge. This suggests that the expression of an antigen as a chimeric protein with CD154 can qualitatively alter immune responses in cattle. Since we previously showed that plasmid encoding tgD-CD154 induces significantly enhanced secondary tgD-specific antibody responses in sheep, there appear to be interspecies differences in the immune responses induced by tgD-CD154, which suggests that both proteins in the chimeric molecule may influence protein targeting and the induction of an immune response.
Figures







References
-
- Saffery R, Choo KH. Strategies for engineering human chromosomes with therapeutic potential. J Gene Med. 2002;4:5–13. - PubMed
-
- Pachuk CJ, McCallus DE, Weiner DB, Satishchandran C. DNA vaccines – challenges in delivery. Curr Opin Mol Ther. 2000;2:188–98. - PubMed
-
- Babiuk LA, Pontarollo R, Babiuk S, Loehr B, van Drunen Littel-van den Hurk S. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003;21:649–58. - PubMed
-
- Pasquini S, Xiang Z, Wang Y, He Z, Deng H, Blaszczyk-Thurin M, Ertl HC. Cytokines and costimulatory molecules as genetic adjuvants. Immunol Cell Biol. 1997;75:397–401. - PubMed
-
- Boyle JS, Brady JL, Lew AM. Enhanced responses to a DNA vaccine encoding a fusion antigen that is directed to sites of immune induction. Nature. 1998;392:408–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
