Sulindac derivatives inhibit cell growth and induce apoptosis in primary cells from malignant peripheral nerve sheath tumors of NF1-patients
- PMID: 15147581
- PMCID: PMC425591
- DOI: 10.1186/1475-2867-4-4
Sulindac derivatives inhibit cell growth and induce apoptosis in primary cells from malignant peripheral nerve sheath tumors of NF1-patients
Abstract
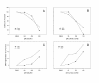
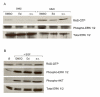
BACKGROUND: Malignant peripheral nerve sheath tumors (MPNSTs) are neoplasms leading to death in most cases. Patients with Neurofibromatosis type 1 have an increased risk of developing this malignancy. The metabolites of the inactive prodrug Sulindac, Sulindac Sulfide and Sulindac Sulfone (Exisulind) are new chemopreventive agents that show promising results in the treatment of different cancer types. In this study we examined the antineoplastic effect of these compounds on primary cells derived from two MPNSTs of Neurofibromatosis type 1 patients. RESULTS: Exisulind and Sulindac Sulfide showed a dramatic time- and dose-dependent growth inhibitory effect with IC50-values of 120 microM and 63 microM, respectively. The decrease in viability of the tested cells correlated with induction of apoptosis. Treatment with 500 microM Exisulind and 125 microM Sulindac Sulfide for a period of 2 days increased the rate of apoptosis 21-27-fold compared to untreated cells. Reduced expression of RAS-GTP and phosphorylated ERK1/2 was detected in treated MPNST cells. Moreover, elevated levels of phosphorylated SAPK/JNK were found after drug treatment, and low activation of cleaved caspase-3 was seen. CONCLUSIONS: Our results suggest that this class of compounds may be of therapeutic benefit for Neurofibromatosis type 1 patients with MPNST.
Figures
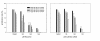



Similar articles
-
Sulindac derivatives inhibit growth and induce apoptosis in human prostate cancer cell lines.Biochem Pharmacol. 1999 Oct 1;58(7):1097-107. doi: 10.1016/s0006-2952(99)00200-2. Biochem Pharmacol. 1999. PMID: 10484067
-
Sulindac sulfide and exisulind inhibit expression of the estrogen and progesterone receptors in human breast cancer cells.Clin Cancer Res. 2006 Jun 1;12(11 Pt 1):3478-84. doi: 10.1158/1078-0432.CCR-05-2051. Clin Cancer Res. 2006. PMID: 16740773
-
NF1 deficiency causes Bcl-xL upregulation in Schwann cells derived from neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors.Int J Oncol. 2013 Feb;42(2):657-66. doi: 10.3892/ijo.2012.1751. Epub 2012 Dec 24. Int J Oncol. 2013. PMID: 23292448
-
Exisulind: Aptosyn, FGN 1, Prevatac, sulindac sulfone.Drugs R D. 2004;5(4):220-6. doi: 10.2165/00126839-200405040-00007. Drugs R D. 2004. PMID: 15230629 Review.
-
Sulindac and its derivatives: a novel class of anticancer agents.Curr Opin Investig Drugs. 2001 May;2(5):677-83. Curr Opin Investig Drugs. 2001. PMID: 11569947 Review.
Cited by
-
CytoregR inhibits growth and proliferation of human adenocarcinoma cells via induction of apoptosis.J Carcinog. 2006 Jan 9;5:1. doi: 10.1186/1477-3163-5-1. J Carcinog. 2006. PMID: 16401338 Free PMC article.
-
Radioprotective Potential of Sulindac Sulfide to Prevent DNA Damage Due to Ionizing Radiation.Drug Des Devel Ther. 2019 Dec 6;13:4127-4134. doi: 10.2147/DDDT.S218022. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31827319 Free PMC article.
-
The role of the immune system in neurofibromatosis type 1-associated nervous system tumors.CNS Oncol. 2017 Jan;6(1):45-60. doi: 10.2217/cns-2016-0024. Epub 2016 Dec 21. CNS Oncol. 2017. PMID: 28001089 Free PMC article. Review.
-
Ral overactivation in malignant peripheral nerve sheath tumors.Mol Cell Biol. 2009 Jul;29(14):3964-74. doi: 10.1128/MCB.01153-08. Epub 2009 May 4. Mol Cell Biol. 2009. PMID: 19414599 Free PMC article.
-
Cancer of the Peripheral Nerve in Neurofibromatosis Type 1.Neurotherapeutics. 2017 Apr;14(2):298-306. doi: 10.1007/s13311-017-0518-y. Neurotherapeutics. 2017. PMID: 28349408 Free PMC article. Review.
References
-
- Gutmann DH, Collins FS. Neurofibromatosis type 1. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editor. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 1995. pp. 76–96.
-
- Riccardi VM. Neurofibromatosis: Phenotype, Natural History and Pathogenesis. The Johns Hopkins University Press, Baltimore. 1992.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

