4-Chloro-benzo[F]isoquinoline (CBIQ) activates CFTR chloride channels and KCNN4 potassium channels in Calu-3 human airway epithelial cells
- PMID: 15148241
- PMCID: PMC1574981
- DOI: 10.1038/sj.bjp.0705846
4-Chloro-benzo[F]isoquinoline (CBIQ) activates CFTR chloride channels and KCNN4 potassium channels in Calu-3 human airway epithelial cells
Abstract
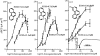
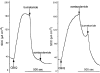
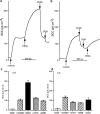
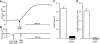
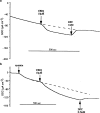
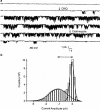
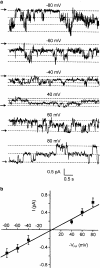
1 Calu-3 cells have been used to investigate the actions of 4-chloro-benzo[F]isoquinoline (CBIQ) on short-circuit current (SCC) in monolayers, whole-cell recording from single cells and by patch clamping. 2 CBIQ caused a sustained, reversible and repeatable increase in SCC in Calu-3 monolayers with an EC50 of 4.0 microm. Simultaneous measurements of SCC and isotopic fluxes of 36Cl- showed that CBIQ caused electrogenic chloride secretion. 3 Apical membrane permeabilisation to allow recording of basolateral membrane conductance in the presence of a K+ gradient suggested that CBIQ activated the intermediate-conductance calcium-sensitive K(+)-channel (KCNN4). Permeabilisation of the basolateral membranes of epithelial monolayers in the presence of a Cl- gradient suggested that CBIQ activated the Cl(-)-channel CFTR in the apical membrane. 4 Whole-cell recording in the absence of ATP/GTP of Calu-3 cells showed that CBIQ generated an inwardly rectifying current sensitive to clotrimazole. In the presence of the nucleotides, a more complex I/V relation was found that was partially sensitive to glibenclamide. The data are consistent with the presence of both KCNN4 and CFTR in Calu-3. 5 Isolated inside-out patches from Calu-3 cells revealed clotrimazole-sensitive channels with a conductance of 12 pS at positive potentials after activation with CBIQ and demonstrating inwardly rectifying properties, consistent with the known properties of KCNN4. Cell-attached patches showed single channel events with a conductance of 7 pS and a linear I/V relation that were further activated by CBIQ by an increase in open state probability, consistent with known properties of CFTR. It is concluded that CBIQ activates CFTR and KCNN4 ion channels in Calu-3 cells.
Figures



References
-
- BECQ F., METTEY Y., GRAY M.A., GALIETTA L.J.V., DORMER R.L., MERTON M., METAYE M., CHAPPE V., MARVINGT-MOUNIR C., ZEGARRA-MORAN O., TARRAN R., BULTEAU L., DERAND R., PEREIRA M.M.C., MCPHERSON M.A., ROGIER C., JOFFRE M., ARGENT B.E., SARROUILHE D., KAMMOUNI W., FIGARELLA C., VERRIER B., GOLA M., VIERFOND J.M. Development of substituted benzo[c]quinolizinium compounds as novel activators of the cystic fibrosis chloride channel. J. Biol. Chem. 1999;274:27415–27425. - PubMed
-
- CACI E., FOLLI C., ZEGARRA-MORAN O., MA T., SPRINGSTEEL M.F., SAMMELSON R.E., NANTZ M.H., KURTH M.J., VERKMAN A.S., GALIETTA L.J.V. CFTR activation in human bronchial epithelial cells by novel benzoflavone and benimidazolone compounds. Am. J. Physiol. 2003;285:L180–L188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

