Nitric oxide-dependent modulation of the delayed rectifier K+ current and the L-type Ca2+ current by ginsenoside Re, an ingredient of Panax ginseng, in guinea-pig cardiomyocytes
- PMID: 15148247
- PMCID: PMC1574975
- DOI: 10.1038/sj.bjp.0705814
Nitric oxide-dependent modulation of the delayed rectifier K+ current and the L-type Ca2+ current by ginsenoside Re, an ingredient of Panax ginseng, in guinea-pig cardiomyocytes
Abstract
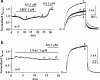
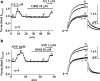
1 Ginsenoside Re, a major ingredient of Panax ginseng, protects the heart against ischemia-reperfusion injury by shortening action potential duration (APD) and thereby prohibiting influx of excessive Ca2+. Ginsenoside Re enhances the slowly activating component of the delayed rectifier K+ current (IKs) and suppresses the L-type Ca2+ current (I(Ca,L)), which may account for APD shortening. 2 We used perforated configuration of patch-clamp technique to define the mechanism of enhancement of IKs and suppression of I(Ca,L) by ginsenoside Re in guinea-pig ventricular myocytes. 3 S-Methylisothiourea (SMT, 1 microm), an inhibitor of nitric oxide (NO) synthase (NOS), and N-acetyl-L-cystein (LNAC, 1 mm), an NO scavenger, inhibited IKs enhancement. Application of an NO donor, sodium nitroprusside (SNP, 1 mm), enhanced IKs with a magnitude similar to that by a maximum dose (20 microm) of ginseonside Re, and subsequent application of ginsenoside Re failed to enhance IKs. Conversely, after IKs had been enhanced by ginsenoside Re (20 microm), subsequently applied SNP failed to further enhance IKs. 4 An inhibitor of guanylate cyclase, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10 microm), barely suppressed IKs enhancement, while a thiol-alkylating reagent, N-ethylmaleimide (NEM, 0.5 mm), clearly suppressed it. A reducing reagent, di-thiothreitol (DTT, 5 mm), reversed both ginsenoside Re- and SNP-induced IKs enhancement. 5 I(Ca,L) suppression by ginsenoside Re (3 microm) was abolished by SMT (1 microm) or LNAC (1 mm). NEM (0.5 mm) did not suppress I(Ca,L) inhibition and DTT (5 mm) did not reverse I(Ca,L) inhibition, whereas in the presence of ODQ (10 microm), ginsenoside Re (3 microm) failed to suppress I(Ca,L). 6 These results indicate that ginsenoside Re-induced IKs enhancement and I(Ca,L) suppression involve NO actions. Direct S-nitrosylation of channel protein appears to be the main mechanism for IKs enhancement, while a cGMP-dependent pathway is responsible for I(Ca,L) inhibition.
Figures

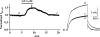
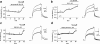
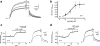
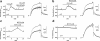

References
-
- ATTELE A.S., WU J.A., YUAN C.-S. Ginseng pharmacology. Multiple constituents and multiple actions. Biochem. Pharmacol. 1999;58:1685–1693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

