Identification of the active metabolite of ticlopidine from rat in vitro metabolites
- PMID: 15148251
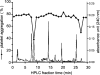
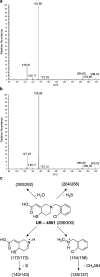
- PMCID: PMC1574971
- DOI: 10.1038/sj.bjp.0705808
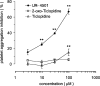
Identification of the active metabolite of ticlopidine from rat in vitro metabolites
Abstract
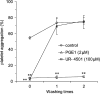
1 Ticlopidine is a well-known anti-platelet agent, but is not active by itself in vitro. We identified a metabolite with anti-platelet activity, which was generated after incubation of 2-oxo-ticlopidine with phenobarbital-induced rat liver homogenate in vitro. 2 An active moiety (UR-4501) was isolated by high-performance liquid chromatography after large-scale preparation of metabolites. 3 The chemical structure of UR-4501 was determined by a combination of liquid chromatography mass/mass spectrometry (LC/MS/MS) and nuclear magnetic resonance (NMR) analysis. 4 UR-4501 produced a concentration-dependent inhibition (3-100 microm) of ADP (10 microm)-induced human platelet aggregation, whereas 2-oxo-ticlopidine (3-100 microm) did not elicit inhibitory responses. 5 UR-4501 (10-100 microm) strongly inhibited ADP- and collagen-induced aggregation and slightly inhibited thrombin-induced aggregation. 6 The inhibition of rat washed platelet aggregation by UR-4501 (100 microm) persisted, even after the platelets had been washed twice. 7 These results suggest that UR-4501 is the molecule responsible for the in vivo activities of ticlopidine.
Figures

Similar articles
-
Identification and biological activity of the active metabolite of clopidogrel.Thromb Haemost. 2000 Nov;84(5):891-6. Thromb Haemost. 2000. PMID: 11127873
-
Inhibition of rat platelet aggregation by mycalolide-B, a novel inhibitor of actin polymerization with a different mechanism of action from cytochalasin-D.Thromb Haemost. 1998 Mar;79(3):614-9. Thromb Haemost. 1998. PMID: 9531051
-
Repeat oral dosing of prasugrel, a novel P2Y12 receptor inhibitor, results in cumulative and potent antiplatelet and antithrombotic activity in several animal species.Eur J Pharmacol. 2008 Jan 28;579(1-3):276-82. doi: 10.1016/j.ejphar.2007.10.005. Epub 2007 Oct 11. Eur J Pharmacol. 2008. PMID: 17996866
-
P2Y12, a new platelet ADP receptor, target of clopidogrel.Semin Vasc Med. 2003 May;3(2):113-22. doi: 10.1055/s-2003-40669. Semin Vasc Med. 2003. PMID: 15199474 Review.
-
Ticlopidine.2020 Sep 25. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. 2020 Sep 25. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. PMID: 31643369 Free Books & Documents. Review.
Cited by
-
Mutational analysis of residues important for ligand interaction with the human P2Y(12) receptor.Eur J Pharmacol. 2010 Oct 10;644(1-3):10-6. doi: 10.1016/j.ejphar.2010.06.023. Epub 2010 Jun 30. Eur J Pharmacol. 2010. PMID: 20599922 Free PMC article.
-
The P2Y12 antagonists, 2-methylthioadenosine 5'-monophosphate triethylammonium salt and cangrelor (ARC69931MX), can inhibit human platelet aggregation through a Gi-independent increase in cAMP levels.J Biol Chem. 2009 Jun 12;284(24):16108-16117. doi: 10.1074/jbc.M809780200. Epub 2009 Apr 3. J Biol Chem. 2009. PMID: 19346255 Free PMC article.
-
A Comparative Study of Molecular Structure, pKa, Lipophilicity, Solubility, Absorption and Polar Surface Area of Some Antiplatelet Drugs.Int J Mol Sci. 2016 Mar 19;17(3):388. doi: 10.3390/ijms17030388. Int J Mol Sci. 2016. PMID: 27007371 Free PMC article.
-
The P2Y(12) receptor as a target of antithrombotic drugs.Purinergic Signal. 2011 Sep;7(3):325-32. doi: 10.1007/s11302-011-9241-z. Epub 2011 Jun 28. Purinergic Signal. 2011. PMID: 21710143 Free PMC article. No abstract available.
-
Complex Cytochrome P450 Kinetics Due to Multisubstrate Binding and Sequential Metabolism. Part 2. Modeling of Experimental Data.Drug Metab Dispos. 2021 Dec;49(12):1100-1108. doi: 10.1124/dmd.121.000554. Epub 2021 Sep 9. Drug Metab Dispos. 2021. PMID: 34503953 Free PMC article.
References
-
- CATTANEO M., AKKAWAT B., LECCHI A., CIMMINIELLO C., CAPITANIO A.M., MANNUCCI P.M. Ticlopidine selectively inhibits human platelet responses to adenosine diphosphate. Thromb. Haemost. 1991;66:694–699. - PubMed
-
- CATTANEO M., WINOCOUR P.D., SOMERS D.A., GROVES H.M., KINLOUGH-RATHBONE R.L., PACKHAM M.A., MUSTARD J.F. Effect of ticlopidine on platelet aggregation, adherence to damaged vessels, thrombus formation and platelet survival. Thromb. Res. 1985;37:29–43. - PubMed
-
- CLARKE T.A., WASKELL L.A. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab Dispos. 2003;31:53–59. - PubMed
-
- DANIEL J.L., DANGELMAIER C., JIN J., ASHBY B., SMITH J.B., KUNAPULI S.P. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J. Biol. Chem. 1998;273:2024–2029. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

