Piracetam and TRH analogues antagonise inhibition by barbiturates, diazepam, melatonin and galanin of human erythrocyte D-glucose transport
- PMID: 15148255
- PMCID: PMC1574967
- DOI: 10.1038/sj.bjp.0705798
Piracetam and TRH analogues antagonise inhibition by barbiturates, diazepam, melatonin and galanin of human erythrocyte D-glucose transport
Abstract
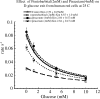
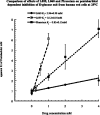
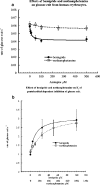
1 Nootropic drugs increase glucose uptake into anaesthetised brain and into Alzheimer's diseased brain. Thyrotropin-releasing hormone, TRH, which has a chemical structure similar to nootropics increases cerebellar uptake of glucose in murine rolling ataxia. This paper shows that nootropic drugs like piracetam (2-oxo 1 pyrrolidine acetamide) and levetiracetam and neuropeptides like TRH antagonise the inhibition of glucose transport by barbiturates, diazepam, melatonin and endogenous neuropeptide galanin in human erythrocytes in vitro. 2 The potencies of nootropic drugs in opposing scopolamine-induced memory loss correlate with their potencies in antagonising pentobarbital inhibition of erythrocyte glucose transport in vitro (P<0.01). Less potent nootropics, D-levetiracetam and D-pyroglutamate, have higher antagonist Ki's against pentobarbital inhibition of glucose transport than more potent L-stereoisomers (P<0.001). 3 Piracetam and TRH have no direct effects on net glucose transport, but competitively antagonise hypnotic drug inhibition of glucose transport. Other nootropics, like aniracetam and levetiracetam, while antagonising pentobarbital action, also inhibit glucose transport. Analeptics like bemigride and methamphetamine are more potent inhibitors of glucose transport than antagonists of hypnotic action on glucose transport. 4 There are similarities between amino-acid sequences in human glucose transport protein isoform 1 (GLUT1) and the benzodiazepine-binding domains of GABAA (gamma amino butyric acid) receptor subunits. Mapped on a 3D template of GLUT1, these homologies suggest that the site of diazepam and piracetam interaction is a pocket outside the central hydrophilic pore region. 5 Nootropic pyrrolidone antagonism of hypnotic drug inhibition of glucose transport in vitro may be an analogue of TRH antagonism of galanin-induced narcosis.
Figures


 DM235 (Ghelardini et al., 2002);
DM235 (Ghelardini et al., 2002);  CG370 (Ogasawara et al., 1995);
CG370 (Ogasawara et al., 1995);  Cyclo (pro-gly) (Seredenin et al., 2002);
Cyclo (pro-gly) (Seredenin et al., 2002);  TRH (Brooks et al., 1987);
TRH (Brooks et al., 1987);  (Abou-Khalil et al., 2003; Brooks et al., 1987; De Reuck & Van Vleymen, 1999; Devinsky & Elger, 2003);
(Abou-Khalil et al., 2003; Brooks et al., 1987; De Reuck & Van Vleymen, 1999; Devinsky & Elger, 2003);  Piracetam (Koskiniemi et al., 1998; De Reuck & Van Vleymen, 1999; Genton et al., 1999);
Piracetam (Koskiniemi et al., 1998; De Reuck & Van Vleymen, 1999; Genton et al., 1999);  Aniracetam (Spignoli & Pepeu, 1987);
Aniracetam (Spignoli & Pepeu, 1987); 
 L060 (Noyer et al., 1995). The in vivo potencies of the drugs were obtained from literature reports of the i.p. dose required to observe a significant nootropic response against scopolamine-induced memory loss. The curvilinearity of the regression line is introduced by using the logarithmic axes required to show the full range of the regression. The regression line is weighted to the errors of the antagonist Ki's of the drugs using the software package in Kaleidagraph 3.6.
L060 (Noyer et al., 1995). The in vivo potencies of the drugs were obtained from literature reports of the i.p. dose required to observe a significant nootropic response against scopolamine-induced memory loss. The curvilinearity of the regression line is introduced by using the logarithmic axes required to show the full range of the regression. The regression line is weighted to the errors of the antagonist Ki's of the drugs using the software package in Kaleidagraph 3.6.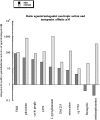

Similar articles
-
Human erythrocyte sugar transport is incompatible with available carrier models.Biochemistry. 1996 Aug 13;35(32):10411-21. doi: 10.1021/bi953077m. Biochemistry. 1996. PMID: 8756697
-
Interactions of ATP, oestradiol, genistein and the anti-oestrogens, faslodex (ICI 182780) and tamoxifen, with the human erythrocyte glucose transporter, GLUT1.Biochem J. 2002 Aug 1;365(Pt 3):707-19. doi: 10.1042/BJ20011624. Biochem J. 2002. PMID: 12133004 Free PMC article.
-
[Participation of GABA--benzodiazepine receptor complex in the anxiolytic effect of piracetam].Eksp Klin Farmakol. 2006 May-Jun;69(3):7-9. Eksp Klin Farmakol. 2006. PMID: 16878489 Russian.
-
Piracetam--an old drug with novel properties?Acta Pol Pharm. 2005 Sep-Oct;62(5):405-9. Acta Pol Pharm. 2005. PMID: 16459490 Review.
-
Piracetam and levetiracetam: close structural similarities but different pharmacological and clinical profiles.Epileptic Disord. 2000 Jun;2(2):99-105. Epileptic Disord. 2000. PMID: 10954241 Review.
Cited by
-
Pharmacokinetic Drug-Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients.Int J Mol Sci. 2021 Sep 3;22(17):9582. doi: 10.3390/ijms22179582. Int J Mol Sci. 2021. PMID: 34502487 Free PMC article. Review.
-
The metabolic enhancer piracetam ameliorates the impairment of mitochondrial function and neurite outgrowth induced by beta-amyloid peptide.Br J Pharmacol. 2010 May;160(2):246-57. doi: 10.1111/j.1476-5381.2010.00656.x. Epub 2010 Mar 9. Br J Pharmacol. 2010. PMID: 20218980 Free PMC article.
-
Pharmacological characterization of DM232 (unifiram) and DM235 (sunifiram), new potent cognition enhancers.CNS Drug Rev. 2006 Spring;12(1):39-52. doi: 10.1111/j.1527-3458.2006.00039.x. CNS Drug Rev. 2006. PMID: 16834757 Free PMC article. Review.
-
Piracetam improves mitochondrial dysfunction following oxidative stress.Br J Pharmacol. 2006 Jan;147(2):199-208. doi: 10.1038/sj.bjp.0706459. Br J Pharmacol. 2006. PMID: 16284628 Free PMC article.
-
Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer's disease--therapeutic aspects.Mol Neurobiol. 2010 Jun;41(2-3):159-71. doi: 10.1007/s12035-010-8141-5. Epub 2010 May 12. Mol Neurobiol. 2010. PMID: 20461558 Review.
References
-
- ABOU-KHALIL B., HEMDAL P., PRIVITERA M.D. An open-label study of levetiracetam at individualised doses between 1000 and 3000 mg day(−1) in adult patients with refractory epilepsy. Seizure. 2003;12:141–149. - PubMed
-
- ADKINS C.E., PILLAI G.V., KERBY J., BONNERT T.P., HALDON C., MCKERNAN R.M., GONZALEZ J.E., OADES K., WHITING P.J., SIMPSON P.B. Alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J. Biol. Chem. 2001;276:38934–38939. - PubMed
-
- BENNETT G.W., BALLARD T.M., WATSON C.D., FONE K.C. Effect of neuropeptides on cognitive function. Exp. Gerontol. 1997;32:451–469. - PubMed
-
- BING O., MOLLER C., ENGEL J.A., SODERPALM B., HEILIG M. Anxiolytic-like action of centrally administered galanin. Neurosci. Lett. 1993;164:17–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

