Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist
- PMID: 15148260
- PMCID: PMC1574962
- DOI: 10.1038/sj.bjp.0705792
Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist
Abstract
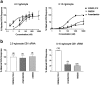
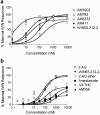
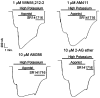
1 The relationship of agonist efficacy to the rate of G protein-coupled receptor signaling desensitization is controversial. 2 Expressing inwardly rectifying potassium channels (GIRKs) in Xenopus oocytes, we have devised a signaling assay that clearly identifies CB1 cannabinoid receptor agonists with low intrinsic efficacy. 3 In this assay, the synthetic CB1 agonists, AM411, AM782, AM1902, AM2233 and WIN55,212-2 and the endogenous cannabinoid, 2-arachidonoyl ester, were full agonists. 4 The synthetic CB1 agonist AM356 (methanandamide), the endogenous cannabinoids, anandamide and 2-arachidonoyl ether, and the phytocannabinoid, Delta9THC, were partial agonists. 5 The rate of desensitization of CB1 was independent of agonist efficacy. WIN55,212-2, AM782, AM1902, AM2233, and 2-arachidonoyl glycerol ester all desensitized quickly, with desensitization rates varying from 14% min(-1) to 10% min(-1). AM356, AM411, anandamide, and Delta9THC all desensitized considerably slower, at a rate of 5% min(-1). 6 Despite high potency and efficacy, AM411 desensitized as slowly as anandamide and Delta9THC. 7 CB1 agonist efficacy and rate of desensitization are not necessarily related.
Figures


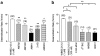
References
-
- BREIVOGEL C.S., SELLEY D.E., CHILDERS S.R. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPgammaS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J. Biol. Chem. 1998;273:16865–16873. - PubMed
-
- DASCAL N. The use of Xenopus oocytes for the study of ion channels. CRC Crit. Rev. Biochem. 1987;22:317–387. - PubMed
-
- DUTTAROY A., YOBURN B.C. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. - PubMed
-
- FAN F., COMPTON D.R., WARD S., MELVIN L., MARTIN B.R. Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J. Pharmacol. Exp. Ther. 1994;271:1383–1390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

