Influence of sodium substitutes on 5-HT-mediated effects at mouse 5-HT3 receptors
- PMID: 15148263
- PMCID: PMC1574959
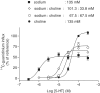
- DOI: 10.1038/sj.bjp.0705788
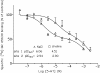
Influence of sodium substitutes on 5-HT-mediated effects at mouse 5-HT3 receptors
Abstract
1 The influence of sodium ion substitutes on the 5-hydroxytryptamine (5-HT)-induced flux of the organic cation [14C]guanidinium through the ion channel of the mouse 5-HT3 receptor and on the competition of 5-HT with the selective 5-HT3 receptor antagonist [3H]GR 65630 was studied, unless stated otherwise, in mouse neuroblastoma N1E-115 cells. 2 Under physiological conditions (135 mm sodium), 5-HT induced a concentration-dependent [14C]guanidinium influx with an EC50 (1.3 microm) similar to that in electrophysiological studies. 3 The stepwise replacement of sodium by increasing concentrations of the organic cation hydroxyethyl trimethylammonium (choline) concentration dependently caused both a rightward shift of the 5-HT concentration-response curve and an increase in the maximum effect of 5-HT. Complete replacement of sodium resulted in a 34-fold lower potency of 5-HT and an almost two times higher maximal response. A low potency of 5-HT in choline buffer was also observed in other 5-HT3 receptor-expressing rodent cell lines (NG 108-15 or NCB 20). 4 Replacement of Na+ by Li+ left the potency and maximal effects of 5-HT almost unchanged. Replacement by tris (hydroxymethyl) methylamine (Tris), tetramethylammonium (TMA) or N-methyl-d-glucamine (NMDG) caused an increase in maximal response to 5-HT similar to that caused by choline. The potency of 5-HT was only slightly reduced by Tris, to a high degree decreased by TMA (comparable to the decrease by choline), but not influenced by NMDG. 5 The potency of 5-HT in inhibiting [3H]GR65630 binding to intact cells was 35-fold lower when sodium was completely replaced by choline, but remained unchanged after replacement by NMDG. 6 The results are compatible with the suggestion that choline competes with 5-HT for the 5-HT3 receptor; the increase in maximal response may be partly due to a choline-mediated delay of the 5-HT-induced desensitization. For studies of 5-HT-evoked [14C]guanidinium flux through 5-HT3 receptor channels, NMDG appears to be an 'ideal' sodium substituent since it increases the signal-to-noise ratio without interfering with 5-HT binding.
Figures



Similar articles
-
Inhibition by steroids of [14C]-guanidinium flux through the voltage-gated sodium channel and the cation channel of the 5-HT3 receptor of N1E-115 neuroblastoma cells.Naunyn Schmiedebergs Arch Pharmacol. 1999 Sep;360(3):234-41. doi: 10.1007/s002109900089. Naunyn Schmiedebergs Arch Pharmacol. 1999. PMID: 10543423
-
Pharmacological differences and similarities between the native mouse 5-HT3 receptor in N1E-115 cells and a cloned short splice variant of the mouse 5-HT3 receptor expressed in HEK 293 cells.Naunyn Schmiedebergs Arch Pharmacol. 1999 Sep;360(3):225-33. doi: 10.1007/s002109900088. Naunyn Schmiedebergs Arch Pharmacol. 1999. PMID: 10543422
-
Characterization of 5-HT3 receptors of N1E-115 neuroblastoma cells by use of the influx of the organic cation [14C]-guanidinium.Br J Pharmacol. 1993 Feb;108(2):436-42. doi: 10.1111/j.1476-5381.1993.tb12822.x. Br J Pharmacol. 1993. PMID: 7680594 Free PMC article.
-
Serotonin 5-HT3 and 5-HT4 ligands: an update of medicinal chemistry research in the last few years.Curr Med Chem. 2010;17(4):334-62. doi: 10.2174/092986710790192730. Curr Med Chem. 2010. PMID: 20015043 Review.
-
5-HT3 receptors.Curr Pharm Des. 2006;12(28):3615-30. doi: 10.2174/138161206778522029. Curr Pharm Des. 2006. PMID: 17073663 Free PMC article. Review.
Cited by
-
Serotonin and beyond-a tribute to Manfred Göthert (1939-2019).Naunyn Schmiedebergs Arch Pharmacol. 2021 Sep;394(9):1829-1867. doi: 10.1007/s00210-021-02083-5. Epub 2021 May 15. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33991216 Free PMC article. Review.
-
The Traditional Japanese Medicine Rikkunshito Promotes Gastric Emptying via the Antagonistic Action of the 5-HT(3) Receptor Pathway in Rats.Evid Based Complement Alternat Med. 2011;2011:248481. doi: 10.1093/ecam/nep173. Epub 2011 Feb 13. Evid Based Complement Alternat Med. 2011. PMID: 19861508 Free PMC article.
References
-
- AAPRO M.S. 5-HT3 receptor antagonists. An overview of their present status and future potential in cancer therapy-induced emesis. Drugs. 1991;42:551–568. - PubMed
-
- BARANN M., DILGER J.P., BÖNISCH H., GÖTHERT M., DYBEK A., URBAN B.W. Inhibition of 5-HT3 receptors by propofol: equilibrium and kinetic measurements. Neuropharmacology. 2000;39:1064–1074. - PubMed
-
- BARANN M., GÖTHERT M., BÖNISCH H., DYBEK A., URBAN B. 5-HT3 receptors in outside-out patches of N1E-115 neuroblastoma cells: Basic properties and effects of pentobarbital. J. Neuropharmacol. 1997;36:655–664. - PubMed
-
- BARANN M., GÖTHERT M., FINK K., BÖNISCH H. Inhibition by anesthetics of 14C-guanidinium flux through the voltage-gated sodium channel and the cation channel of the 5-HT3 receptor of N1E-115 neuroblastoma cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:125–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

