T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis
- PMID: 15148335
- PMCID: PMC2211818
- DOI: 10.1084/jem.20032196
T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis
Abstract
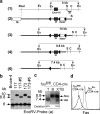
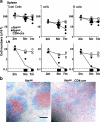
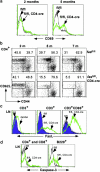
To study the role of Fas-Fas ligand (FasL) interaction-mediated apoptosis in lymphocyte homeostasis, we generated a mutant fas allele allowing conditional inactivation of the fas gene through Cre-mediated recombination. Experiments in which Fas was ablated in T cells, B cells, T and B cells, or in a more generalized manner demonstrated that the development of lymphoproliferative disease as seen in Fas-deficient mice requires Fas ablation in lymphoid and nonlymphoid tissues. Selective inactivation of Fas in T cells led to a severe lymphopenia over time, accompanied by up-regulation of FasL on activated T cells and apoptosis of peripheral lymphocytes. In addition, the mutant animals developed a fatal wasting syndrome caused by massive leukocyte infiltration in the lungs together with increased inflammatory cytokine production and pulmonary fibrosis. Inhibition of Fas-FasL interaction in vivo completely prevented the loss of lymphocytes and initial lymphocyte infiltration in the lungs. Thus, FasL-mediated interaction of activated, Fas-deficient T cells with Fas-expressing cells in their environment leads to break down of lymphocyte homeostasis and development of a lung disease strikingly resembling idiopathic pulmonary fibrosis in humans, a common and severe disease for which the mutant mice may serve as a first animal model.
Figures
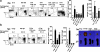


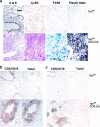
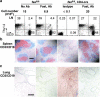
References
-
- Krammer, P.H. 1999. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv. Immunol. 71:163–210. - PubMed
-
- Krammer, P.H. 2000. CD95's deadly mission in the immune system. Nature. 407:789–795. - PubMed
-
- Watanabe-Fukunaga, R., C.I. Brannan, N.G. Copeland, N.A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 356:314–317. - PubMed
-
- Takahashi, T., M. Tanaka, C.I. Brannan, N.A. Jenkins, N.G. Copeland, T. Suda, and S. Nagata. 1994. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 76:969–976. - PubMed
-
- Rieux-Laucat, F., F. Le Deist, C. Hivroz, I.A. Roberts, K.M. Debatin, A. Fischer, and J.P. de Villartay. 1995. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 268:1347–1349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

