Gene delivery by dendrimers operates via a cholesterol dependent pathway
- PMID: 15148360
- PMCID: PMC419601
- DOI: 10.1093/nar/gkh595
Gene delivery by dendrimers operates via a cholesterol dependent pathway
Abstract
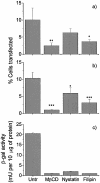
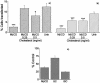
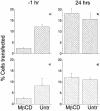
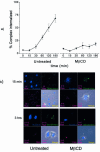
Understanding the cellular uptake and intracellular trafficking of dendrimer-DNA complexes is an important prerequisite for improving the transfection efficiency of non-viral vector-mediated gene delivery. Dendrimers are synthetic polymers used for gene transfer. Although these cationic molecules show promise as versatile DNA carriers, very little is known about the mechanism of gene delivery. This paper investigates how the uptake occurs, using an endothelial cell line as model, and evaluates whether the internalization of dendriplexes takes place randomly on the cell surface or at preferential sites such as membrane rafts. Following extraction of plasma membrane cholesterol, the transfection efficiency of the gene delivered by dendrimers was drastically decreased. Replenishment of membrane cholesterol restored the gene expression. The binding and especially internalization of dendriplexes was strongly reduced by cholesterol depletion before transfection. However, cholesterol removal after transfection did not inhibit expression of the delivered gene. Fluorescent dendriplexes co-localize with the ganglioside GM1 present into membrane rafts in both an immunoprecipitation assay and confocal microscopy studies. These data strongly suggest that membrane cholesterol and raft integrity are physiologically relevant for the cellular uptake of dendrimer-DNA complexes. Hence these findings provide evidence that membrane rafts are important for the internalization of non-viral vectors in gene therapy.
Figures



Similar articles
-
Effects of the nanostructure of dendrimer/DNA complexes on their endocytosis and gene expression.Biomaterials. 2010 Jul;31(21):5660-70. doi: 10.1016/j.biomaterials.2010.03.059. Biomaterials. 2010. PMID: 20399497
-
Fas signaling induces raft coalescence that is blocked by cholesterol depletion in human RPE cells undergoing apoptosis.Invest Ophthalmol Vis Sci. 2006 May;47(5):2172-8. doi: 10.1167/iovs.05-1167. Invest Ophthalmol Vis Sci. 2006. PMID: 16639029
-
Role of lipid rafts in E-cadherin-- and HGF-R/Met--mediated entry of Listeria monocytogenes into host cells.J Cell Biol. 2004 Aug 30;166(5):743-53. doi: 10.1083/jcb.200406078. J Cell Biol. 2004. PMID: 15337781 Free PMC article.
-
P-Glycoprotein is localized in intermediate-density membrane microdomains distinct from classical lipid rafts and caveolar domains.FEBS J. 2005 Oct;272(19):4924-37. doi: 10.1111/j.1742-4658.2005.04905.x. FEBS J. 2005. PMID: 16176266
-
How to study dendriplexes II: Transfection and cytotoxicity.J Control Release. 2010 Jan 25;141(2):110-27. doi: 10.1016/j.jconrel.2009.09.030. Epub 2009 Oct 6. J Control Release. 2010. PMID: 19815039 Review.
Cited by
-
[Comparison of several viral vectors for gene therapy of corneal endothelial cells].Ophthalmologe. 2005 Dec;102(12):1168-74. doi: 10.1007/s00347-005-1230-6. Ophthalmologe. 2005. PMID: 15886987 German.
-
Uptake and intracellular traffic of siRNA dendriplexes in glioblastoma cells and macrophages.Int J Nanomedicine. 2011;6:2715-28. doi: 10.2147/IJN.S25235. Epub 2011 Nov 4. Int J Nanomedicine. 2011. PMID: 22114502 Free PMC article.
-
Nanovehicular intracellular delivery systems.J Pharm Sci. 2008 Sep;97(9):3518-90. doi: 10.1002/jps.21270. J Pharm Sci. 2008. PMID: 18200527 Free PMC article. Review.
-
Integrin targeted delivery of gene therapeutics.Theranostics. 2011 Mar 2;1:211-9. doi: 10.7150/thno/v01p0211. Theranostics. 2011. PMID: 21547161 Free PMC article.
-
The mechanism of polyplex internalization into cells: testing the GM1/caveolin-1 lipid raft mediated endocytosis pathway.Mol Pharm. 2010 Feb 1;7(1):267-79. doi: 10.1021/mp900241t. Mol Pharm. 2010. PMID: 20025295 Free PMC article.
References
-
- Tang M.X., Redemann,C.T. and Szoka,F.C.,Jr (1996) In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem., 7, 703–714. - PubMed
-
- Ramaswamy C., Sakthivel,T., Wilderspin,A.F. and Florence,A.T. (2003) Dendriplexes and their characterisation. Int. J. Pharm., 254, 17–21. - PubMed
-
- Bielinska A.U., Kukowska-Latallo,J.F. and Baker,J.R.,Jr (1997) The interaction of plasmid DNA with polyamidoamine dendrimers: mechanism of complex formation and analysis of alterations induced in nuclease sensitivity and transcriptional activity of the complexed DNA. Biochim. Biophys. Acta, 1353, 180–190. - PubMed
-
- Heiniger H.J., Kandutsch,A.A. and Chen,H.W. (1976) Depletion of L-cell sterol depresses endocytosis. Nature, 263, 515–517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

