Trigger factor binds to ribosome-signal-recognition particle (SRP) complexes and is excluded by binding of the SRP receptor
- PMID: 15148364
- PMCID: PMC419529
- DOI: 10.1073/pnas.0402231101
Trigger factor binds to ribosome-signal-recognition particle (SRP) complexes and is excluded by binding of the SRP receptor
Abstract
Trigger factor (TF) and signal recognition particle (SRP) bind to the bacterial ribosome and are both crosslinked to protein L23 at the peptide exit, where they interact with emerging nascent peptide chains. It is unclear whether TF and SRP exclude one another from their ribosomal binding site(s). Here we show that SRP and TF can bind simultaneously to ribosomes or ribosome nascent-chain complexes exposing a SRP-specific signal sequence. Based on changes of the crosslinking pattern and on results obtained by fluorescence measurements using fluorescence-labeled SRP, TF binding induces structural changes in the ribosome-SRP complex. Furthermore, we show that binding of the SRP receptor, FtsY, to ribosome-bound SRP excludes TF from the ribosome. These results suggest that TF and SRP sample nascent chains on the ribosome in a nonexclusive fashion. The decision for ribosome nascent-chain complexes exposing a signal sequence to enter SRP-dependent membrane targeting seems to be determined by the binding of SRP, which is stabilized by signal sequence recognition, and promoted by the exclusion of TF due to the binding of the SRP receptor to ribosome-bound SRP.
Figures



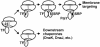
References
-
- Ban, N., Nissen, P., Hansen, J., Moore, P. B. & Steitz, T. A. (2000) Science 289, 905–920. - PubMed
-
- Harms, J., Schluenzen, F., Zarivach, R., Bashan, A., Gat, S., Agmon, I., Bartels, H., Franceschi, F. & Yonath, A. (2001) Cell 107, 679–688. - PubMed
-
- Frydman, J. (2001) Annu. Rev. Biochem. 70, 603–647. - PubMed
-
- Hartl, F. U. & Hayer-Hartl, M. (2002) Science 295, 1852–1858. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

