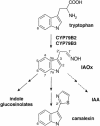
Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis
- PMID: 15148388
- PMCID: PMC419588
- DOI: 10.1073/pnas.0305876101
Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis
Abstract
Characteristic for cruciferous plants is their production of N- and S-containing indole phytoalexins with disease resistance and cancer-preventive properties, previously proposed to be synthesized from indole independently of tryptophan. We show that camalexin, the indole phytoalexin of Arabidopsis thaliana, is synthesized from tryptophan via indole-3-acetaldoxime (IAOx) in a reaction catalyzed by CYP79B2 and CYP79B3. Cyp79B2/cyp79B3 double knockout mutant is devoid of camalexin, as it is also devoid of indole glucosinolates [Zhao, Y., Hull, A. K., Gupta, N. R., Goss, K. A., Alonso, J., Ecker, J. R., Normanly, J., Chory, J. & Celenza, J. L. (2002) Genes Dev. 16, 3100-3112], and isotope-labeled IAOx is incorporated into camalexin. These results demonstrate that only CYP79B2 and CYP79B3 contribute significantly to the IAOx pool from which camalexin and indole glucosinolates are synthesized. Furthermore, production of camalexin in the sur1 mutant devoid of glucosinolates excludes the possibility that camalexin is derived from indole glucosinolates. CYP79B2 plays an important role in camalexin biosynthesis in that the transcript level of CYP79B2, but not CYP79B3, is increased upon induction of camalexin by silver nitrate as evidenced by microarray analysis and promoter-beta-glucuronidase data. The structural similarity between cruciferous indole phytoalexins suggests that these compounds are biogenetically related and synthesized from tryptophan via IAOx by CYP79B homologues. The data show that IAOx is a key branching point between several secondary metabolic pathways as well as primary metabolism, where IAOx has been shown to play a critical role in IAA homeostasis.
Figures
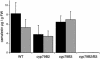
References
-
- Pedras, M. S. C., Okanga, F. I., Zaharia, I. L. & Khan, A. Q. (2000) Phytochemistry 53, 161–176. - PubMed
-
- Glawischnig, E., Mikkelsen, M. D. & Halkier, B. A. (2003) in Sulphur in Plants, eds. Abrol, Y. & Ahmad, A. (Kluwer, Dordrecht, The Netherlands), pp. 145–162.
-
- Mezencev, R., Mojzis, J., Pilatova, M. & Kutschy, P. (2003) Neoplasma (Bratisl.) 50, 239–245. - PubMed
-
- Hanley, A. B., Parsley, K. R., Lewis, J. A. & Fenwick, G. R. (1990) J. Chem. Soc. Perkin Trans. 1 , 2273–2276.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

