Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles
- PMID: 15148394
- PMCID: PMC419540
- DOI: 10.1073/pnas.0308045101
Membrane growth can generate a transmembrane pH gradient in fatty acid vesicles
Abstract
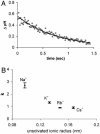
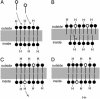
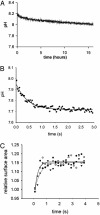
Electrochemical proton gradients are the basis of energy transduction in modern cells, and may have played important roles in even the earliest cell-like structures. We have investigated the conditions under which pH gradients are maintained across the membranes of fatty acid vesicles, a model of early cell membranes. We show that pH gradients across such membranes decay rapidly in the presence of alkali-metal cations, but can be maintained in the absence of permeable cations. Under such conditions, when fatty acid vesicles grow through the incorporation of additional fatty acid, a transmembrane pH gradient is spontaneously generated. The formation of this pH gradient captures some of the energy released during membrane growth, but also opposes and limits further membrane area increase. The coupling of membrane growth to energy storage could have provided a growth advantage to early cells, once the membrane composition had evolved to allow the maintenance of stable pH gradients.
Figures

References
-
- Walde, P., Wick, R., Fresta, M., Mangone, A. & Luisi, P. L. (1994) J. Am. Chem. Soc. 116, 11649–11654.
-
- Berclaz, N., Muller, M., Walde, P. & Luisi, P. L. (2001) J. Phys. Chem. B 105, 1056–1064.
-
- Szathmary, E. & Demeter, L. (1987) J. Theor. Biol. 128, 463–486. - PubMed
-
- Chakrabarti, A. C., Breaker, R. R., Joyce, G. F. & Deamer, D. W. (1994) J. Mol. Evol. 39, 555–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

