A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase
- PMID: 15148399
- PMCID: PMC419532
- DOI: 10.1073/pnas.0400664101
A new activity for an old enzyme: Escherichia coli bacterial alkaline phosphatase is a phosphite-dependent hydrogenase
Abstract
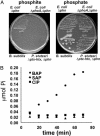
Genetic analysis indicates that Escherichia coli possesses two independent pathways for oxidation of phosphite (Pt) to phosphate. One pathway depends on the 14-gene phn operon, which encodes the enzyme C-P lyase. The other pathway depends on the phoA locus, which encodes bacterial alkaline phosphatase (BAP). Transposon mutagenesis studies strongly suggest that BAP is the only enzyme involved in the phoA-dependent pathway. This conclusion is supported by purification and biochemical characterization of the Pt-oxidizing enzyme, which was proven to be BAP by N terminus protein sequencing. Highly purified BAP catalyzed Pt oxidation with specific activities of 62-242 milliunits/mg and phosphate ester hydrolysis with specific activities of 41-61 units/mg. Surprisingly, BAP catalyzes the oxidation of Pt to phosphate and molecular H2. Thus, BAP is a unique Pt-dependent, H2-evolving hydrogenase. This reaction is unprecedented in both P and H biochemistry, and it is likely to involve direct transfer of hydride from the substrate to water-derived protons.
Figures


Similar articles
-
Characterization of heterodimeric alkaline phosphatases from Escherichia coli: an investigation of intragenic complementation.J Mol Biol. 2000 Dec 8;304(4):645-56. doi: 10.1006/jmbi.2000.4230. J Mol Biol. 2000. PMID: 11099386
-
Hydrogen-oxidizing hydrogenases 1 and 2 of Escherichia coli regulate the onset of hydrogen evolution and ATPase activity, respectively, during glucose fermentation at alkaline pH.FEMS Microbiol Lett. 2013 Nov;348(2):143-8. doi: 10.1111/1574-6968.12281. Epub 2013 Oct 10. FEMS Microbiol Lett. 2013. PMID: 24111652
-
Two C-P lyase operons in Pseudomonas stutzeri and their roles in the oxidation of phosphonates, phosphite, and hypophosphite.J Bacteriol. 2004 Jul;186(14):4730-9. doi: 10.1128/JB.186.14.4730-4739.2004. J Bacteriol. 2004. PMID: 15231805 Free PMC article.
-
Structure and mechanism of alkaline phosphatase.Annu Rev Biophys Biomol Struct. 1992;21:441-83. doi: 10.1146/annurev.bb.21.060192.002301. Annu Rev Biophys Biomol Struct. 1992. PMID: 1525473 Review.
-
Mechanism and applications of phosphite dehydrogenase.Bioorg Chem. 2005 Jun;33(3):171-89. doi: 10.1016/j.bioorg.2005.01.003. Epub 2005 Feb 23. Bioorg Chem. 2005. PMID: 15888310 Review.
Cited by
-
Effects of Mulberry Leaf Extract on the Liver Function of Juvenile Spotted Sea Bass (Lateolabrax maculatus).Aquac Nutr. 2023 Oct 23;2023:2892463. doi: 10.1155/2023/2892463. eCollection 2023. Aquac Nutr. 2023. PMID: 37908498 Free PMC article.
-
Plausible Emergence and Self Assembly of a Primitive Phospholipid from Reduced Phosphorus on the Primordial Earth.Orig Life Evol Biosph. 2021 Sep;51(3):185-213. doi: 10.1007/s11084-021-09613-4. Epub 2021 Jul 19. Orig Life Evol Biosph. 2021. PMID: 34279769 Review.
-
Probing the origin of the compromised catalysis of E. coli alkaline phosphatase in its promiscuous sulfatase reaction.J Am Chem Soc. 2007 May 2;129(17):5760-5. doi: 10.1021/ja069111+. Epub 2007 Apr 6. J Am Chem Soc. 2007. PMID: 17411045 Free PMC article.
-
The Streptomyces-produced antibiotic fosfomycin is a promiscuous substrate for archaeal isopentenyl phosphate kinase.Biochemistry. 2012 Jan 31;51(4):917-25. doi: 10.1021/bi201662k. Epub 2012 Jan 11. Biochemistry. 2012. PMID: 22148590 Free PMC article.
-
Characterization of phoA, a Bacterial Alkaline Phosphatase for Phi Use Efficiency in Rice Plant.Front Plant Sci. 2019 Feb 25;10:37. doi: 10.3389/fpls.2019.00037. eCollection 2019. Front Plant Sci. 2019. PMID: 30858852 Free PMC article.
References
-
- Ternan, N. G., McGrath, J. W., McMullan, G. & Quinn, J. P. (1998) World J. Microbiol. Biotech. 14, 635–647.
-
- Seto, H. a. K., Tomohisa. (1999) Nat. Prod. Rep. 16, 589–596. - PubMed
-
- Schink, B. & Friedrich, M. (2000) Nature 406, 37. - PubMed
-
- Schink, B., Thiemann, V., Laue, H. & Friedrich, M. W. (2002) Arch. Microbiol. 177, 381–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

