Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors
- PMID: 15148402
- PMCID: PMC419578
- DOI: 10.1073/pnas.0401080101
Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors
Abstract
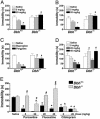
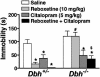
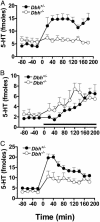
Mice unable to synthesize norepinephrine (NE) and epinephrine due to targeted disruption of the dopamine beta-hydroxylase gene, Dbh, were used to critically test roles for NE in mediating acute behavioral changes elicited by different classes of antidepressants. To this end, we used the tail suspension test, one of the most widely used paradigms for assessing antidepressant activity and depression-related behaviors in normal and genetically modified mice. Dbh(-/-) mice failed to respond to the behavioral effects of various antidepressants, including the NE reuptake inhibitors desipramine and reboxetine, the monoamine oxidase inhibitor pargyline, and the atypical antidepressant bupropion, even though they did not differ in baseline immobility from Dbh(+/-) mice, which have normal levels of NE. Surprisingly, the effects of the selective serotonin reuptake inhibitors (SSRIs) fluoxetine, sertraline, and paroxetine were also absent or severely attenuated in the Dbh(-/-) mice. In contrast, citalopram (the most selective SSRI) was equally effective at reducing immobility in mice with and without NE. Restoration of NE by using L-threo-3,4-dihydroxyphenylserine reinstated the behavioral effects of both desipramine and paroxetine in Dbh(-/-) mice, thus demonstrating that the reduced sensitivity to antidepressants is related to NE function, as opposed to developmental abnormalities resulting from chronic NE deficiency. Microdialysis studies demonstrated that the ability of fluoxetine to increase hippocampal serotonin was blocked in Dbh(-/-) mice, whereas citalopram's effect was only partially attenuated. These data show that NE plays an important role in mediating acute behavioral and neurochemical actions of many antidepressants, including most SSRIs.
Figures
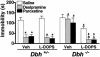
Similar articles
-
Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test.Psychopharmacology (Berl). 2007 Jun;192(3):357-71. doi: 10.1007/s00213-007-0728-9. Epub 2007 Feb 21. Psychopharmacology (Berl). 2007. PMID: 17318507
-
Use of dopamine-beta-hydroxylase-deficient mice to determine the role of norepinephrine in the mechanism of action of antidepressant drugs.J Pharmacol Exp Ther. 2001 Aug;298(2):651-7. J Pharmacol Exp Ther. 2001. PMID: 11454927
-
Hypobaric hypoxia exposure in rats differentially alters antidepressant efficacy of the selective serotonin reuptake inhibitors fluoxetine, paroxetine, escitalopram and sertraline.Pharmacol Biochem Behav. 2018 Jul;170:25-35. doi: 10.1016/j.pbb.2018.05.002. Epub 2018 May 5. Pharmacol Biochem Behav. 2018. PMID: 29738811
-
Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding.Cell Mol Neurobiol. 1999 Aug;19(4):467-89. doi: 10.1023/a:1006986824213. Cell Mol Neurobiol. 1999. PMID: 10379421 Free PMC article. Review.
-
The pharmacogenetics of the selective serotonin reuptake inhibitors.Clin Investig. 1993 Dec;71(12):1002-9. doi: 10.1007/BF00180032. Clin Investig. 1993. PMID: 8124052 Review.
Cited by
-
Dopamine beta-hydroxylase deficiency.Orphanet J Rare Dis. 2006 Mar 30;1:7. doi: 10.1186/1750-1172-1-7. Orphanet J Rare Dis. 2006. PMID: 16722595 Free PMC article. Review.
-
Local extracellular K+ in cortex regulates norepinephrine levels, network state, and behavioral output.Proc Natl Acad Sci U S A. 2023 Oct 3;120(40):e2305071120. doi: 10.1073/pnas.2305071120. Epub 2023 Sep 29. Proc Natl Acad Sci U S A. 2023. PMID: 37774097 Free PMC article.
-
Blue again: perturbational effects of antidepressants suggest monoaminergic homeostasis in major depression.Front Psychol. 2011 Jul 7;2:159. doi: 10.3389/fpsyg.2011.00159. eCollection 2011. Front Psychol. 2011. PMID: 21779273 Free PMC article.
-
The interaction of escitalopram and R-citalopram at the human serotonin transporter investigated in the mouse.Psychopharmacology (Berl). 2014 Dec;231(23):4527-40. doi: 10.1007/s00213-014-3595-1. Epub 2014 May 9. Psychopharmacology (Berl). 2014. PMID: 24810106 Free PMC article.
-
Depressive-like behavior observed with a minimal loss of locus coeruleus (LC) neurons following administration of 6-hydroxydopamine is associated with electrophysiological changes and reversed with precursors of norepinephrine.Neuropharmacology. 2016 Feb;101:76-86. doi: 10.1016/j.neuropharm.2015.09.003. Epub 2015 Sep 8. Neuropharmacology. 2016. PMID: 26362360 Free PMC article.
References
-
- Frazer, A. (1997) J. Clin. Psychiatry 58, Suppl. 6, 9–25. - PubMed
-
- Stanford, S. C. (1996) Trends Pharmacol. Sci. 17, 150–154. - PubMed
-
- Owens, M. J., Knight, D. L. & Nemeroff, C. B. (2000) Biol. Psychiatry 47, 842–845. - PubMed
-
- Millan, M. J., Gobert, A., Lejeune, F., Newman-Tancredi, A., Rivet, J-M., Auclair, A. & Peglion, J-L. (2001) J. Pharmacol. Exp. Ther. 298, 565–580. - PubMed
-
- Nakayama, K. (2002) Naunyn-Schmiedeberg's Arch. Pharmacol. 365, 102–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

