The mechanism of transmembrane S-nitrosothiol transport
- PMID: 15148403
- PMCID: PMC419527
- DOI: 10.1073/pnas.0401167101
The mechanism of transmembrane S-nitrosothiol transport
Abstract
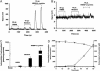
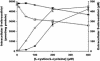
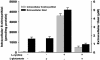
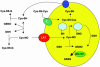
S-nitrosothiols have been suggested to play an important role in nitric oxide (NO)-mediated biological events. However, the mechanisms by which an S-nitrosothiol (or the S-nitroso functional group) is transferred across cell membrane are still poorly understood. We have demonstrated previously that the degradation of S-nitrosoglutathione (GSNO) by cells absolutely required the presence of cystine in the extracellular medium and proposed a mechanism that involved the reduction of cystine to cysteine, followed by the reaction of cysteine with GSNO to form S-nitrosocysteine (CysNO), mixed disulfides, and nitrosyl anion. In the present study we have assessed the effect of cystine on the transfer of the S-nitroso functional group from the extracellular to the intracellular space. Using RAW 264.7 cells, we found that the presence of L-cystine enhanced GSNO-dependent S-nitrosothiol uptake, increasing the intracellular S-nitrosothiol level from approximately 60 pmol/mg of protein to approximately 3 nmol/mg of protein. The uptake seems to depend on the reduction of L-cystine to L-cysteine, which involves the xc- amino acid transport system, the transnitrosation between GSNO and L-cysteine to form L-CysNO, and uptake of L-CysNO via amino acid transport system L. Compared with GSNO, (Z)-1-[N-(3-ammoniopropyl)-N-[4-(3-aminopropylammonio)butyl]-amino]diazen-1-ium-1,2-diolate, an NO donor, is much less effective at intracellular S-nitrosothiol formation in the presence of L-cystine or L-cysteine, suggesting that the biochemical changes that occur after exposure of cells to S-nitrosothiol, with respect to thiol chemistry, are distinctly different from those observed with NO.
Figures
References
-
- Pawloski, J. R., Hess, D. T. & Stamler, J. S. (2001) Nature 409, 622–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

