Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria
- PMID: 15148411
- PMCID: PMC419544
- DOI: 10.1073/pnas.0401897101
Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria
Abstract
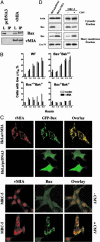
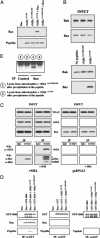
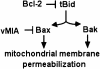
We report that the cytomegalovirus-encoded cell death suppressor vMIA binds Bax and prevents Bax-mediated mitochondrial membrane permeabilization by sequestering Bax at mitochondria in the form of a vMIA-Bax complex. vMIA mutants with a defective mitochondria-targeting domain retain their Bax-binding function but not their ability to suppress mitochondrial membrane permeabilization or cell death. vMIA does not seem to either specifically associate with Bak or suppress Bak-mediated mitochondrial membrane permeabilization. Recent evidence suggests that the contribution of Bax and Bak in the mitochondrial apoptotic signaling pathway depends on the distinct phenotypes of cells, and it appears from our data that vMIA is capable of suppressing apoptosis in cells in which this pathway is dominated by Bax, but not in cells where Bak also plays a role.
Figures

References
-
- McCormick, A. L., Skaletskaya, A., Barry, P. A., Mocarski, E. S. & Goldmacher, V. S. (2003) Virology 316, 221–233. - PubMed
-
- Goldmacher, V. S. (2002) Biochimie 84, 177–185. - PubMed
-
- Yang, J., Liu, X., Bhalla, K., Kim, C. N., Ibrado, A. M., Cai, J., Peng, T. I., Jones, D. P. & Wang, X. (1997) Science 275, 1129–1132. - PubMed
-
- Kluck, R. M., Bossy-Wetzel, E., Green, D. R. & Newmeyer, D. D. (1997) Science 275, 1132–1136. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

