The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence
- PMID: 15150220
- PMCID: PMC415768
- DOI: 10.1128/JB.186.11.3355-3362.2004
The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence
Abstract
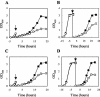
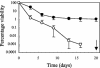
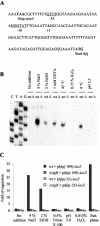
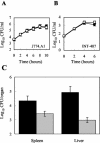
In gram-negative bacteria, the RNA-binding protein Hfq has emerged as an important regulatory factor in a variety of physiological processes, including stress resistance and virulence. In Escherichia coli, Hfq modulates the stability or the translation of mRNAs and interacts with numerous small regulatory RNAs. Here, we studied the role of Hfq in the stress tolerance and virulence of the gram-positive food-borne human pathogen Listeria monocytogenes. We present evidence that Hfq is involved in the ability of L. monocytogenes to tolerate osmotic and ethanol stress and contributes to long-term survival under amino acid-limiting conditions. However, Hfq is not required for resistance to acid and oxidative stress. Transcription of hfq is induced under various stress conditions, including osmotic and ethanol stress and at the entry into the stationary growth phase, thus supporting the view that Hfq is important for the growth and survival of L. monocytogenes in harsh environments. The stress-inducible transcription of hfq depends on the alternative sigma factor sigmaB, which controls the expression of numerous stress- and virulence-associated genes in L. monocytogenes. Infection studies showed that Hfq contributes to pathogenesis in mice, yet plays no role in the infection of cultured cell lines. This study provides, for the first time, information on the role of Hfq in the stress tolerance and virulence of a gram-positive pathogen.
Figures

References
-
- Brøndsted, L., B. H. Kallipolitis, H. Ingmer, and S. Knöchel. 2003. kdpE and a putative RsbQ homologue contribute to growth of Listeria monocytogenes at high osmolarity and low temperature. FEMS Microbiol. Lett. 219:233-239. - PubMed
-
- Ermolaeva, S., T. Karpova, S. Novella, M. Wagner, M. Scortti, I. Tartakovskii, and J. A. Vazquez-Boland. 2003. A simple method for the differentiation of Listeria monocytogenes based on induction of lecithinase activity by charcoal. Int. J. Food Microbiol. 82:87-94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

