The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation
- PMID: 15150404
- PMCID: PMC428477
- DOI: 10.1073/pnas.0402770101
The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation
Erratum in
- Proc Natl Acad Sci U S A. 2006 Jan 10;103(2):504. Grim, Jonathan A [corrected to Grim, Jonathan E]
Abstract
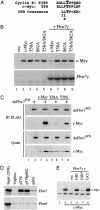
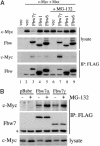
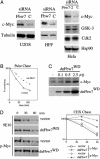
Myc proteins regulate cell growth and division and are implicated in a wide range of human cancers. We show here that Fbw7, a component of the SCF(Fbw7) ubiquitin ligase and a tumor suppressor, promotes proteasome-dependent c-Myc turnover in vivo and c-Myc ubiquitination in vitro. Phosphorylation of c-Myc on threonine-58 (T58) by glycogen synthase kinase 3 regulates the binding of Fbw7 to c-Myc as well as Fbw7-mediated c-Myc degradation and ubiquitination. T58 is the most frequent site of c-myc mutations in lymphoma cells, and our findings suggest that c-Myc activation is one of the key oncogenic consequences of Fbw7 loss in cancer. Because Fbw7 mediates the degradation of cyclin E, Notch, and c-Jun, as well as c-Myc, the loss of Fbw7 is likely to elicit profound effects on cell proliferation during tumorigenesis.
Figures

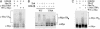
Comment in
-
Myc degradation: dancing with ubiquitin ligases.Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8843-4. doi: 10.1073/pnas.0403046101. Epub 2004 Jun 8. Proc Natl Acad Sci U S A. 2004. PMID: 15187232 Free PMC article. No abstract available.
Similar articles
-
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction.Nature. 2011 Mar 3;471(7336):104-9. doi: 10.1038/nature09732. Nature. 2011. PMID: 21368833 Free PMC article.
-
Regulation of GATA-binding protein 2 levels via ubiquitin-dependent degradation by Fbw7: involvement of cyclin B-cyclin-dependent kinase 1-mediated phosphorylation of THR176 in GATA-binding protein 2.J Biol Chem. 2015 Apr 17;290(16):10368-81. doi: 10.1074/jbc.M114.613018. Epub 2015 Feb 10. J Biol Chem. 2015. PMID: 25670854 Free PMC article.
-
Fbw7 promotes ubiquitin-dependent degradation of c-Myb: involvement of GSK3-mediated phosphorylation of Thr-572 in mouse c-Myb.Oncogene. 2009 Jun 25;28(25):2393-405. doi: 10.1038/onc.2009.111. Epub 2009 May 4. Oncogene. 2009. PMID: 19421138
-
Tumor suppressor activities of the Fbw7 E3 ubiquitin ligase receptor.Cancer Biol Ther. 2005 May;4(5):506-8. doi: 10.4161/cbt.4.5.1703. Epub 2005 May 5. Cancer Biol Ther. 2005. PMID: 15908780 Review.
-
Aberrant regulation of FBW7 in cancer.Oncotarget. 2014 Apr 30;5(8):2000-15. doi: 10.18632/oncotarget.1859. Oncotarget. 2014. PMID: 24899581 Free PMC article. Review.
Cited by
-
Critical roles of Myc-ODC axis in the cellular transformation induced by myeloproliferative neoplasm-associated JAK2 V617F mutant.PLoS One. 2013;8(1):e52844. doi: 10.1371/journal.pone.0052844. Epub 2013 Jan 3. PLoS One. 2013. PMID: 23300995 Free PMC article.
-
c-Myc plays a key role in IFN-γ-induced persistence of Chlamydia trachomatis.Elife. 2022 Sep 26;11:e76721. doi: 10.7554/eLife.76721. Elife. 2022. PMID: 36155135 Free PMC article.
-
A Complex Interplay between Wnt/β-Catenin Signalling and the Cell Cycle in the Adult Liver.Int J Hepatol. 2012;2012:816125. doi: 10.1155/2012/816125. Epub 2012 Sep 3. Int J Hepatol. 2012. PMID: 22973520 Free PMC article.
-
Guttiferone K impedes cell cycle re-entry of quiescent prostate cancer cells via stabilization of FBXW7 and subsequent c-MYC degradation.Cell Death Dis. 2016 Jun 2;7(6):e2252. doi: 10.1038/cddis.2016.123. Cell Death Dis. 2016. PMID: 27253416 Free PMC article.
-
Mutant BRAF Upregulates MCL-1 to Confer Apoptosis Resistance that Is Reversed by MCL-1 Antagonism and Cobimetinib in Colorectal Cancer.Mol Cancer Ther. 2016 Dec;15(12):3015-3027. doi: 10.1158/1535-7163.MCT-16-0017. Epub 2016 Oct 7. Mol Cancer Ther. 2016. PMID: 27765849 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

