Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer
- PMID: 15150570
- PMCID: PMC2409523
- DOI: 10.1038/sj.bjc.6601879
Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer
Abstract
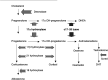
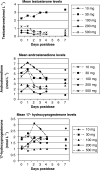
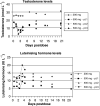
A series of three dose escalating studies were conducted to investigate the ability of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate, to cause maximum suppression of testosterone synthesis when delivered to castrate and noncastrate males with prostate cancer. Study A was a single dose study in castrate males. Study B was a single dose study in noncastrate males and study C was a multiple dose study in noncastrate males. The drug was given orally in a once-daily dose and blood samples taken to assess pharmacokinetic (PK) parameters and hormone levels in all patients. The study drug was well tolerated with some variability in PKs. Suppression of testosterone levels to <0.14 nmol l(-1) was seen in four out of six castrate males treated with a single dose of 500 mg. At 800 mg given days 1-12 in noncastrate males, target suppression was achieved in three out of three patients, but a two- to three-fold increase of Luteinising Hormone (LH) levels in two out of three patients overcame suppression within 3 days. All patients in the multiple dose study developed an abnormal response to a short Synacthen test by day 11, although baseline cortisol levels remained normal. This is the first report of the use of a specific 17alpha-hydroxylase/(17,20)-lyase inhibitor in humans. Repeated treatment of men with intact gonadal function with abiraterone acetate at a dose of 800 mg can successfully suppress testosterone levels to the castrate range. However, this level of suppression may not be sustained in all patients due to compensatory hypersecretion of LH. The enhanced testosterone suppression achieved in castrate men merits further clinical study as a second-line hormonal treatment for prostate cancer. Adrenocortical suppression may necessitate concomitant administration of replacement glucocorticoid.
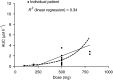
Figures

References
-
- Barrie SE, Haynes BP, Potter GA, Chan FCY, Goddard PM, Dowsett M, Jarman M (1997) Biochemistry and Pharmacokinetics of Potent Non-steroidal Cytochrome P45017α Inhibitors. J Steroid Biochem Mol Biol 60(5/6): 347–351 - PubMed
-
- Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M (1994) Pharmacology of novel steroidal inhibitors of cytochrome P45017α (17α-hydroxylase/C17-20 lyase). J Steroid Biochem Mol Biol 50(5/6): 267–273 - PubMed
-
- Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser T, Willi N, Mihatsch M, Sauter G, Kallioniemi O (1999) Survey of gene amplifications during prostate cancer progression by high-throughput fluorescence in situ hybridization on tissue microarrays. Cancer Res 59: 803–806 - PubMed
-
- Cancer Research UK (2002) Combined statistics from Mortality Statistics Cause DH 2 Nat Stat (Eng & W), Registrar General for Scotland Annual Report, Registrar General for Nth Ire Annual Report May 2002
-
- DeMario MD, Ratain MJ (1998) Oral chemotherapy: rationale and future directions. J Clin Oncol 16(7): 2557–2567 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

