Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer
- PMID: 15150584
- PMCID: PMC2409484
- DOI: 10.1038/sj.bjc.6601856
Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer
Abstract
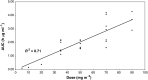
SP1049C is a novel anticancer agent containing doxorubicin and two nonionic pluronic block copolymers. In preclinical studies, SP1049C demonstrated increased efficacy compared to doxorubicin. The objectives of this first phase I study were to determine the toxicity profile, dose-limiting toxicity, maximum tolerated dose and pharmacokinetic profile of SP1049C, and to document any antitumour activity. The starting dose was 5 mg m(-2) (doxorubicin content) as an intravenous infusion once every 3 weeks for up to six cycles. A total of 26 patients received 78 courses at seven dose levels. The dose-limiting toxicity was myelosuppression and DLT was reached at 90 mg m(-2). The maximum tolerated dose was 70 mg m(-2) and is recommended for future trials. The pharmacokinetic profile of SP1049C showed a slower clearance than has been reported for conventional doxorubicin. Evidence of antitumour activity was seen in some patients with advanced resistant solid tumours. Phase II trials with this agent are now warranted to further define its antitumour activity and safety profile.
Figures
Similar articles
-
A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction.Invest New Drugs. 2011 Oct;29(5):1029-37. doi: 10.1007/s10637-010-9399-1. Epub 2010 Feb 24. Invest New Drugs. 2011. PMID: 20179989 Clinical Trial.
-
Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study.Lancet Oncol. 2012 Dec;13(12):1234-41. doi: 10.1016/S1470-2045(12)70476-X. Epub 2012 Nov 13. Lancet Oncol. 2012. PMID: 23153506 Clinical Trial.
-
First-in-man phase I study assessing the safety and pharmacokinetics of a 1-hour intravenous infusion of the doxorubicin prodrug DTS-201 every 3 weeks in patients with advanced or metastatic solid tumours.Eur J Cancer. 2017 Nov;86:240-247. doi: 10.1016/j.ejca.2017.09.009. Epub 2017 Oct 19. Eur J Cancer. 2017. PMID: 29055839 Clinical Trial.
-
Treatment of metastatic breast cancer with paclitaxel and doxorubicin.Semin Oncol. 1995 Dec;22(6 Suppl 15):13-7. Semin Oncol. 1995. PMID: 8643964 Review.
-
Review of docetaxel/doxorubicin combination in metastatic breast cancer.Oncology (Williston Park). 1997 Aug;11(8 Suppl 8):31-3. Oncology (Williston Park). 1997. PMID: 9364539 Review.
Cited by
-
Novel air leak test using surfactant for lung surgery.J Thorac Dis. 2018 Dec;10(12):6472-6474. doi: 10.21037/jtd.2018.11.34. J Thorac Dis. 2018. PMID: 30746190 Free PMC article.
-
The potential of polymeric micelles in the context of glioblastoma therapy.Front Pharmacol. 2013 Dec 30;4:157. doi: 10.3389/fphar.2013.00157. Front Pharmacol. 2013. PMID: 24416018 Free PMC article. Review.
-
Preliminary study on fabrication, characterization and synergistic anti-lung cancer effects of self-assembled micelles of covalently conjugated celastrol-polyethylene glycol-ginsenoside Rh2.Drug Deliv. 2017 Nov;24(1):834-845. doi: 10.1080/10717544.2017.1326540. Drug Deliv. 2017. PMID: 28532223 Free PMC article.
-
The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents.Biomaterials. 2010 Feb;31(5):923-33. doi: 10.1016/j.biomaterials.2009.09.101. Epub 2009 Oct 22. Biomaterials. 2010. PMID: 19853293 Free PMC article.
-
Structural parameters governing activity of Pluronic triblock copolymers in hyperthermia cancer therapy.Int J Hyperthermia. 2011;27(7):663-71. doi: 10.3109/02656736.2011.599828. Int J Hyperthermia. 2011. PMID: 21992559 Free PMC article.
References
-
- Alakhov V, Klinski E, Li S, Pietrzynski G, Venne A, Batrakova E, Bronich T, Kabanov A (1999) Block copolymer-based formulation of doxorubicin. From cell screen to clinical trials. Colloids Surfaces B: Biointerfaces 16: 113–134
-
- Batrakova EV, Dorodonych TY, Klinski EY, Kliushnenkova EN, Shemchukova OB, Goncharova ON, Arjakov SA, Alakhov VY, Kabanov AV (1996) Anthracycline antibiotics, non-covalently incorporated into block copolymer micelles: in vivo evaluation of anti-cancer activity. Br J Cancer 74: 1545–1552 - PMC - PubMed
-
- Bronchud MH, Margison M, Howell A, Lind M, Lucas SB, Wilkinson PM (1990) Comparative pharmacokinetics of escalating doses of doxorubicin in patients with metastatic breast cancer. Cancer Chemother Pharmacol 25: 435–439 - PubMed
-
- Collet JH (2003) Poloxamers. In Handbook of Pharmaceutical Excipients, Rowe RC, Sheskey PJ, Weller PJ (eds) 4th edn. pp 447–450. Washington, DC: American Pharmaceutical Association
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources