Impact of antibody framework residue VH-71 on the stability of a humanised anti-MUC1 scFv and derived immunoenzyme
- PMID: 15150594
- PMCID: PMC2409732
- DOI: 10.1038/sj.bjc.6601759
Impact of antibody framework residue VH-71 on the stability of a humanised anti-MUC1 scFv and derived immunoenzyme
Abstract
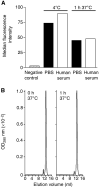
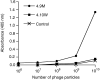
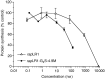
Anti-MUC1 single-chain Fv (scFv) fragments generated from the humanised antibody huHMFG1 had adequate antigen-binding properties but very poor stability irrespective of the applied linker or domain orientation. Mutagenesis of heavy-chain framework residue V(H)-71, previously described as a key residue for maintaining the CDR-H2 main-chain conformation and thus important for antigen binding, markedly stabilised the scFv while having only a minor effect on the binding affinity of the molecule. Because of its improved stability, the engineered fragment exhibited immunoreactivity with tumour cells even after 7 days of incubation in human serum at 37 degrees C. It also showed, in contrast to the wild-type scFv, a concentration-dependent binding to the target antigen when displayed on phage. When fusing the scFv to the recombinant ribonuclease rapLRI, only the fusion protein generated with the stable mutant scFv was able to kill MUC1(+) tumour cells with an IC(50) of 80 nM. We expect this novel immunoenzyme to become a promising tool for the treatment of MUC1(+) malignancies.
Figures




Similar articles
-
Generation of a humanised single chain Fv (Scfv) derived from the monoclonal Eric-1 recognising the human neural cell adhesion molecule.Med Pediatr Oncol. 2001 Jan;36(1):243-6. doi: 10.1002/1096-911X(20010101)36:1<243::AID-MPO1060>3.0.CO;2-5. Med Pediatr Oncol. 2001. PMID: 11464896
-
Generation of a highly stable, internalizing anti-CD22 single-chain Fv fragment for targeting non-Hodgkin's lymphoma.Int J Cancer. 2003 Dec 10;107(5):822-9. doi: 10.1002/ijc.11451. Int J Cancer. 2003. PMID: 14566834
-
Mutual stabilization of VL and VH in single-chain antibody fragments, investigated with mutants engineered for stability.Biochemistry. 1998 Sep 22;37(38):13120-7. doi: 10.1021/bi980712q. Biochemistry. 1998. PMID: 9748318
-
Pharmacokinetics and biodistribution of genetically-engineered antibodies.Q J Nucl Med. 1998 Dec;42(4):225-41. Q J Nucl Med. 1998. PMID: 9973838 Review.
-
[New type recombinant antibody fragment scFv multimer and cancer targeting].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2003 Jun;20(2):361-5. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2003. PMID: 12856620 Review. Chinese.
Cited by
-
Manufacturability and functionality assessment of different formats of T-cell engaging bispecific antibodies.MAbs. 2023 Jan-Dec;15(1):2231129. doi: 10.1080/19420862.2023.2231129. MAbs. 2023. PMID: 37403264 Free PMC article.
-
PEP-Patch: Electrostatics in Protein-Protein Recognition, Specificity, and Antibody Developability.J Chem Inf Model. 2023 Nov 27;63(22):6964-6971. doi: 10.1021/acs.jcim.3c01490. Epub 2023 Nov 7. J Chem Inf Model. 2023. PMID: 37934909 Free PMC article.
-
Mutation of Framework Residue H71 Results in Different Antibody Paratope States in Solution.Front Immunol. 2021 Mar 2;12:630034. doi: 10.3389/fimmu.2021.630034. eCollection 2021. Front Immunol. 2021. PMID: 33737932 Free PMC article.
-
Defining and Studying B Cell Receptor and TCR Interactions.J Immunol. 2023 Aug 1;211(3):311-322. doi: 10.4049/jimmunol.2300136. J Immunol. 2023. PMID: 37459189 Free PMC article.
-
Hiding in plain sight: structure and sequence analysis reveals the importance of the antibody DE loop for antibody-antigen binding.MAbs. 2020 Jan-Dec;12(1):1840005. doi: 10.1080/19420862.2020.1840005. MAbs. 2020. PMID: 33180672 Free PMC article.
References
-
- Aboud-Pirak E, Sergent T, Otte-Slachmuylder C, Abarca J, Trouet A, Schneider YJ (1988) Binding and endocytosis of a monoclonal antibody to a high molecular weight human milk fat globule membrane-associated antigen by cultured MCF-7 breast carcinoma cells. Cancer Res 48: 3188–3196 - PubMed
-
- Adams GP, Schier R, Marshall K, Wolf EJ, McCall AM, Marks JD, Weiner LM (1998) Increased affinity leads to improved selective tumor delivery of single-chain Fv antibodies. Cancer Res 58: 485–490 - PubMed
-
- Arndt MA, Krauss J, Schwarzenbacher R, Vu BK, Greene S, Rybak SM (2003) Generation of a highly stable, internalizing anti-CD22 single-chain Fv fragment for targeting non-Hodgkin's lymphoma. Int J Cancer 107: 822–829 - PubMed
-
- Baldus SE, Monig SP, Hanisch FG, Zirbes TK, Flucke U, Oelert S, Zilkens G, Madejczik B, Thiele J, Schneider PM, Holscher AH, Dienes HP (2002) Comparative evaluation of the prognostic value of MUC1, MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal adenocarcinoma. Histopathology 40: 440–449 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

