Negative human papillomavirus testing in normal smears selects a population at low risk for developing high-grade cervical lesions
- PMID: 15150605
- PMCID: PMC2409748
- DOI: 10.1038/sj.bjc.6601726
Negative human papillomavirus testing in normal smears selects a population at low risk for developing high-grade cervical lesions
Abstract
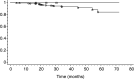
High-risk human papillomaviruses (HR-HPV) are the necessary cause of cervical carcinomas and there is an increasing interest in using HR-HPV DNA detection in adjunction to cytological examination for primary cervical screening. To determine whether women with a normal smear negative for HR-HPV DNA detection with the Hybrid Capture II assay might represent a low-risk population for developing a high-grade squamous intraepithelial lesion (HSIL), 4401 women have been followed in a period of 12-72 months (median=34 months). During this follow-up, four HSIL and one microinvasive carcinoma have been detected in this cohort (three in the cohort of 3526 women >29 years). The global negative predictive value (NPV) of double-negative tests is thus of 99.9% (ninety-five percent confidence interval (95% CI): 99.8-100%), whereas cytology alone gives an NPV of 99.2% (95% CI: 98.9-99.5%). If we obtain a second negative HR-HPV test 1-2 years after the initial test, the NPV is 100%. The NPV is also of 100% in the cohort of women >49 years. We conclude that all these women could be safely screened at longer intervals between 3 and 5 years. This policy will offset the increased costs induced by an additional HR-HPV testing in primary screening.
Figures


References
-
- Bory JP, Cucherousset J, Lorenzato M, Gabriel R, Quereux C, Birembaut P, Clavel C (2002) Recurrent human papillomavirus infection detected by Hybrid Capture II selects women with normal cervical smears at risk for developing high grade cervical lesions. A longitudinal study of 3091 women. Int J Cancer 102: 519–525 - PubMed
-
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen, AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV, International Biological Study in Cervical Cancer Study Group (1995) Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst 87: 796–802 - PubMed
-
- Clavel C, Masure M, Bory JP, Putaud I, Mangeonjean C, Lorenzato M, Gabriel R, Quereux C, Birembaut P (1999) Hybrid Capture II-based human papillomavirus detection, a sensitive test to detect in routine high-grade cervical lesions: a preliminary study on 1518 women. Br J Cancer 80: 1306–1311 - PMC - PubMed
-
- Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ (1995) Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 172: 946–954 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

