Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian Collaborative Study from the Mario Negri Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R. (Istituto Oncologico Romagnolo) group
- PMID: 15150623
- PMCID: PMC2409494
- DOI: 10.1038/sj.bjc.6601787
Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian Collaborative Study from the Mario Negri Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R. (Istituto Oncologico Romagnolo) group
Abstract
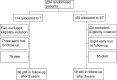
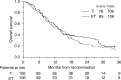
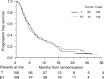
The aim of the study was to evaluate the role of epidoxorubicin plus paclitaxel combination (ET) vs single agent paclitaxel (T), as second-line chemotherapy treatment in advanced ovarian cancer patients in early progression within 12 months after platinum-based chemotherapy. From October 1994 up to June 1999, 234 patients from 34 Italian hospitals were randomised to receive: (A) epidoxorubicin (E) 80 mg m(-2) + paclitaxel (T) 175 mg m(-2) (3 h infusion), every 21 days for 4-6 cycles. (B) Paclitaxel 175 mg m(-2) (3 h infusion) every 21 days for 4-6 cycles. Evaluable for survival analysis were 106 and 106 patients in ET and T arm, respectively. Platinum-based monochemotherapy was the first-line treatment in 43% patients, while polichemotherapy containing anthracyclines was the preferred first-line therapy in 22% patients. The median time from the end of first-line therapy to randomisation was 3 months. Treatment was completed in 87 and 85% of T and ET arm, respectively. Haematological toxicity was significantly more common in ET group (ECOG grade 3-4 neutropenia: 37.4% in ET vs 18.2% in T arm). Neuropathies were similar in both arms (sensory: ECOG grade 2-3: 12.1% in ET vs 14.7% in T arm, motor: 6.1% in ET vs 5.3% in T arm). Objective response was achieved in 37.4% of patients in ET group and in 46.9% of patients in T arm. At a median follow-up of time of 48 months, a total of 180 patients progressed and 163 patients died. Survival analysis showed no difference between ET and T (median time to progression: 6 months for both regimens, median survival: 12 and 14 months for ET and T, respectively; hazard ratio for mortality of ET vs T: 1.17 (95% CI 0.86-1.59; P=0.33). The ET regimen does not seem to be more effective than T in refractory advanced ovarian cancer patients in early progression after platinum-based chemotherapy. Despite an acceptable response rate, the control of disease progression remains poor.
Figures
References
-
- Bolis G, Parazzini F, Scarfone G, Villa A, Amoroso M, Rabaiotti E, Polatti A, Reina S, Piroetti E (1999) Paclitaxel vs epidoxorubicin plus paclitaxel as second-line therapy for platinum-refractory and resistant ovarian cancer. Gynecol Oncol 72: 60–64 - PubMed
-
- Cannistra S (2002) Is there a ‘best’ choice of second-line agent in the treatment of recurrent, potentially platinum-sensitive ovarian cancer? J Clin Oncol 20: 1158–1160 - PubMed
-
- Cantù MG, Buda A, Parma G, Rossi R, Floriani I, Bonazzi C, Dell'Anna T, Torri V Colombo N (2002) Randomized controlled trial of single-agent paclitaxel versus cyclophosphamide, doxorubicin, and cisplatin in patients with recurrent ovarian cancer who responded to first-line platinum-based regimens. J Clin Oncol 20: 1232–1237 - PubMed
-
- Einzig AI, Wiernik PH, Sasloff J, Runowicz CD, Goldberg GL (1992) Phase II and long-term follow-up of patients treated with Paclitaxel for advanced ovarian adenocarcinoma. J Clin Oncol 10: 1748–1753 - PubMed
-
- Eisenhauer EA, Vermorken JB, van Glabbeke M (1997) Predictors response to subsequent chemotherapy in platinum pre-treated ovarian cancer: a multivariate analysis of 704 patients. Ann Oncol 8: 963–968 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical