Phase II study of capecitabine in combination with paclitaxel in patients with anthracycline-pretreated advanced/metastatic breast cancer
- PMID: 15150624
- PMCID: PMC2410278
- DOI: 10.1038/sj.bjc.6601784
Phase II study of capecitabine in combination with paclitaxel in patients with anthracycline-pretreated advanced/metastatic breast cancer
Abstract
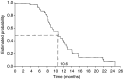
The addition of oral capecitabine to docetaxel improves response rate, time to progression (TTP) and overall survival in anthracycline-pretreated metastatic breast cancer (MBC). This phase II study evaluates the efficacy and safety of a 21-day cycle of oral capecitabine (1000 mg m(-2) twice daily, days 1-14) plus i.v. paclitaxel (175 mg m(-2), day 1) in anthracycline-pretreated advanced/MBC. In all, 73 patients were enrolled at 13 Swedish and Spanish centres. The objective response rate was 52% (95% confidence interval (CI): 40-63%) in the intent-to-treat population, including complete responses in 11%. Disease was stabilised in a further 29%. The median time to disease progression (TTP) was 8.1 months and the median overall survival was 16.5 months. The combination was generally well tolerated with a predictable safety profile. The most common treatment-related nonhaematological adverse events were hand-foot syndrome (42%), alopecia (30%) and diarrhoea (26%). The only treatment-related Grade 3/4 adverse events occurring in >5% of patients were alopecia (22%) and hand-foot syndrome (11%). Grade 3/4 neutropenia and lymphocytopenia were reported in 12 and 14% of patients, respectively. Capecitabine plus paclitaxel is highly active with a favourable safety profile in anthracycline-pretreated MBC.
Figures
Similar articles
-
Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results.J Clin Oncol. 2002 Jun 15;20(12):2812-23. doi: 10.1200/JCO.2002.09.002. J Clin Oncol. 2002. PMID: 12065558 Clinical Trial.
-
Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines.Br J Cancer. 2002 May 6;86(9):1367-72. doi: 10.1038/sj.bjc.6600261. Br J Cancer. 2002. PMID: 11986765 Free PMC article. Clinical Trial.
-
Final results of a phase II clinical trial of weekly docetaxel in combination with capecitabine in anthracycline-pretreated metastatic breast cancer.Clin Breast Cancer. 2004 Oct;5(4):287-92. doi: 10.3816/cbc.2004.n.032. Clin Breast Cancer. 2004. PMID: 15507175 Clinical Trial.
-
Capecitabine: a review of its pharmacology and therapeutic efficacy in the management of advanced breast cancer.Drugs. 2003;63(2):217-36. doi: 10.2165/00003495-200363020-00009. Drugs. 2003. PMID: 12515569 Review.
-
Treatment for anthracycline-pretreated metastatic breast cancer.Oncologist. 2002;7 Suppl 6:4-12. doi: 10.1634/theoncologist.7-suppl_6-4. Oncologist. 2002. PMID: 12454314 Review.
Cited by
-
Efficacy and toxicity of Trastuzumab and Paclitaxel plus Capecitabine in the first-line treatment of HER2-positive metastatic breast cancer.J Cancer Res Clin Oncol. 2013 Jun;139(6):981-6. doi: 10.1007/s00432-013-1409-1. Epub 2013 Mar 5. J Cancer Res Clin Oncol. 2013. PMID: 23463098 Free PMC article. Clinical Trial.
-
Efficacy of the oral fluorouracil pro-drug capecitabine in cancer treatment: a review.Molecules. 2008 Aug 27;13(8):1897-922. doi: 10.3390/molecules13081897. Molecules. 2008. PMID: 18794792 Free PMC article. Review.
-
Paclitaxel, Epirubicin and Capecitabine (TEX) as First-Line Treatment for Metastatic Breast Cancer: a Pilot Phase I/II Feasibility Study.Clin Med Oncol. 2008;2:533-8. doi: 10.4137/cmo.s1027. Epub 2008 Sep 25. Clin Med Oncol. 2008. PMID: 21892328 Free PMC article.
-
Severe skin toxicity observed with the combination of capecitabine and weekly paclitaxel in metastatic breast cancer patients.Support Care Cancer. 2008 Dec;16(12):1415-8. doi: 10.1007/s00520-008-0495-0. Epub 2008 Sep 5. Support Care Cancer. 2008. PMID: 18773227 Clinical Trial.
-
A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer.Br J Cancer. 2008 Jan 29;98(2):316-22. doi: 10.1038/sj.bjc.6604186. Epub 2008 Jan 22. Br J Cancer. 2008. PMID: 18219288 Free PMC article. Clinical Trial.
References
-
- Abrams JS, Vena DA, Baltz J, Adams J, Montello M, Christian M, Onetto N, Desmond-Hellmann S, Canetta R, Friedman MA, Arbuck SG (1995) Paclitaxel activity in heavily pretreated breast cancer: a National Cancer Institute Treatment Referral Center trial. J Clin Oncol 13: 2056–2065 - PubMed
-
- Blum JL (2001) The role of capecitabine, an oral, enzymatically activated fluoropyrimidine, in the treatment of metastatic breast cancer. Oncologist 6: 56–64 - PubMed
-
- Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, Osterwalder B (2001a) Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 92: 1759–1768 - PubMed
-
- Blum JL, Jones SE, Buzdar AU (2001b) Capecitabine in 162 patients with paclitaxel-pretreated MBC: updated results and analysis of dose-modification. Eur J Cancer 37(Suppl 6): 190 (abstr. 693)
-
- Blum JL, Jones SE, Buzdar AU, Lo Russo PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T (1999) Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17: 485–493 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical