Population pharmacokinetics of pyrimethamine and sulfadoxine in children with congenital toxoplasmosis
- PMID: 15151519
- PMCID: PMC1884514
- DOI: 10.1111/j.1365-2125.2004.02077.x
Population pharmacokinetics of pyrimethamine and sulfadoxine in children with congenital toxoplasmosis
Abstract
Aims: To develop a population pharmacokinetic model for pyrimethamine (PYR) and sulfadoxine (SDX) in children with congenital toxoplasmosis.
Methods: Children were treated with PYR (1.25 mg kg(-1)) and SDX (25 mg kg(-1)) (Fansidar) plus folinic acid (Lederfoline) 5 mg). Plasma concentrations, available from a therapeutic drug monitoring database, were determined by high-performance liquid chromatography. Population pharmacokinetic analysis was performed using a nonlinear mixed effects model.
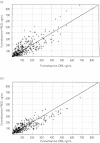
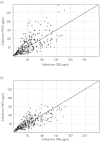
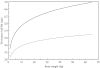
Results: Eighty-nine children, aged 1 week to 14 years and weighing 2.9-59 kg, were available for evaluation. Both PYR and SDX concentration-time profiles were best described by a one-compartment open model. Volume of plasma distribution (V) and clearance (CL) were significantly related to body weight (BW) using an allometric function. Typical CL and V estimates (95% confidence interval), for a child weighing 11 kg were 5.50 (5.28, 5.73) l day(-1) and 36 (33, 39) l for PYR and 0.26 (0.25, 0.27) l day(-1) and 2.1 (1.9, 2.3) l for SDX. For BW between 3.5 and 60 kg, plasma half-lives were predicted to vary from 4.0 to 5.2 days for PYR, and from 5.0 to 7.5 days for SDX.
Conclusion: This study indicated that body weight influences PYR and SDX pharmacokinetics in children. To optimize PYR/SDX combination treatment in congenital toxoplasmosis, short dosing intervals in very young low-wight children are probably appropriate.
Figures
Similar articles
-
Population pharmacokinetics of pyrimethamine and sulfadoxine in children treated for congenital toxoplasmosis.Antimicrob Agents Chemother. 2004 Oct;48(10):3794-800. doi: 10.1128/AAC.48.10.3794-3800.2004. Antimicrob Agents Chemother. 2004. PMID: 15388436 Free PMC article. Clinical Trial.
-
Pharmacokinetic disposition of sulfadoxine in children with acute uncomplicated falciparum malaria treated with sulfadoxine-pyrimethamine in South West Nigeria.Am J Ther. 2012 Sep;19(5):338-45. doi: 10.1097/MJT.0b013e3181baf266. Am J Ther. 2012. PMID: 19918170
-
Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications.Clin Pharmacol Ther. 2006 Dec;80(6):582-96. doi: 10.1016/j.clpt.2006.08.016. Clin Pharmacol Ther. 2006. PMID: 17178260
-
[Treatment of ocular toxoplasmosis. Part 1: Basic principles and diagnosis].Kinderarztl Prax. 1993 May;61(3):90-6. Kinderarztl Prax. 1993. PMID: 8326704 Review. German.
-
[Treatment of toxoplasmosis].Klin Med (Mosk). 1979 May;57(5):21-7. Klin Med (Mosk). 1979. PMID: 384081 Review. Russian. No abstract available.
Cited by
-
Treatment of infants with congenital toxoplasmosis: tolerability and plasma concentrations of sulfadiazine and pyrimethamine.Eur J Pediatr. 2006 Jan;165(1):19-25. doi: 10.1007/s00431-005-1665-4. Epub 2005 Aug 20. Eur J Pediatr. 2006. PMID: 16133245
-
In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone.Antimicrob Agents Chemother. 2008 Apr;52(4):1269-77. doi: 10.1128/AAC.01203-07. Epub 2008 Jan 22. Antimicrob Agents Chemother. 2008. PMID: 18212105 Free PMC article.
-
Residual antimalarials in malaria patients from Tanzania--implications on drug efficacy assessment and spread of parasite resistance.PLoS One. 2009 Dec 14;4(12):e8184. doi: 10.1371/journal.pone.0008184. PLoS One. 2009. PMID: 20011529 Free PMC article.
-
Population pharmacokinetics of chloroquine and sulfadoxine and treatment response in children with malaria: suggestions for an improved dose regimen.Br J Clin Pharmacol. 2008 Apr;65(4):493-501. doi: 10.1111/j.1365-2125.2007.03050.x. Epub 2008 Feb 20. Br J Clin Pharmacol. 2008. PMID: 18294337 Free PMC article. Clinical Trial.
-
Pharmacokinetic properties of sulfadoxine-pyrimethamine in pregnant women.Antimicrob Agents Chemother. 2009 Oct;53(10):4368-76. doi: 10.1128/AAC.00335-09. Epub 2009 Jul 20. Antimicrob Agents Chemother. 2009. PMID: 19620325 Free PMC article. Clinical Trial.
References
-
- Villena I, Aubert D, Leroux B, et al. Pyrimethamine-sulfadoxine treatment of congenital toxoplasmosis: follow-up of 78 cases between 1980 and 1997. Scand J Infect Dis. 1998;30:295–300. - PubMed
-
- Koppe JG, Loewer-Sieger DH, De Roever-Bonnet H. Results of 20 years follow up of congenital toxoplasmosis. Lancet. 1986;1:254–6. - PubMed
-
- Guerina NG. Congenital infection with Toxoplasma gondii. Pediatr Ann. 1994;23:138–51. - PubMed
-
- Harris C, Salgo MP, Tanowitz HB, Wittner M. In vitro assessment of antimicrobial agents against Toxoplasma gondii. J Infect Dis. 1988;157:14–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

