Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn
- PMID: 15152035
- PMCID: PMC6729459
- DOI: 10.1523/JNEUROSCI.5211-03.2004
Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn
Abstract
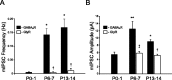
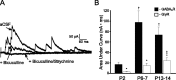
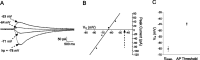
Cutaneous spinal sensory transmission appears to lack inhibitory control in the newborn spinal cord, but the properties of GABAergic and glycinergic synapses in the neonatal dorsal horn have not been characterized. Whole-cell patch-clamp recordings from rat superficial dorsal horn neurons in spinal cord slices at postnatal day 0 (P0) to P2, P6-P7, and P13-P14 revealed an age-dependent increase in the frequency of spontaneous IPSCs, which were abolished by the GABA(A) receptor (GABA(A)R) antagonist bicuculline between P0 and P7 but not at P14. GABA(A)R-mediated miniature IPSCs (mIPSCs), but not glycinergic mIPSCs, were present at birth, and GABA mIPSCs remained more frequent than glycine mIPSCs at all ages. Sciatic nerve stimulation resulted in IPSCs with both GABAergic and glycinergic components, although a larger contribution arose from GABA(A) receptors at all ages. In gramicidin perforated patch-clamp recordings, exogenous GABA applications produced depolarization in 40% of neurons at P0-P2, but the reversal potential of GABA-evoked currents (E(GABA)) was consistently more negative than action potential threshold at this age. By P6-P7, GABA evoked only membrane hyperpolarization. The GABA(B)R agonist baclofen elicited an outward current in all neurons with peak amplitudes observed by P6-P7 and abolished sciatic nerve-evoked monosynaptic glutamatergic EPSCs in all groups. The results show considerable postnatal development of inhibitory processing in the dorsal horn with GABAergic mechanisms initially dominant over glycinergic events. GABA(A)R-mediated depolarizations during the first postnatal week are likely to be important for the maturation of spinal networks but do not provide a major excitatory drive to the newborn dorsal horn.
Figures
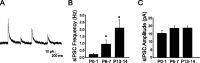
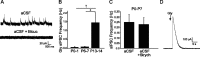

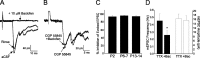

Similar articles
-
Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks.J Neurophysiol. 2001 Jul;86(1):492-502. doi: 10.1152/jn.2001.86.1.492. J Neurophysiol. 2001. PMID: 11431527
-
Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy.J Physiol. 2007 Mar 15;579(Pt 3):849-61. doi: 10.1113/jphysiol.2006.126102. Epub 2007 Jan 11. J Physiol. 2007. PMID: 17218355 Free PMC article.
-
Differential contribution of GABAergic and glycinergic components to inhibitory synaptic transmission in lamina II and laminae III-IV of the young rat spinal cord.Eur J Neurosci. 2007 Nov;26(10):2940-9. doi: 10.1111/j.1460-9568.2007.05919.x. Eur J Neurosci. 2007. PMID: 18001289
-
Developmental Formation of the GABAergic and Glycinergic Networks in the Mouse Spinal Cord.Int J Mol Sci. 2022 Jan 13;23(2):834. doi: 10.3390/ijms23020834. Int J Mol Sci. 2022. PMID: 35055019 Free PMC article. Review.
-
Pre- and postsynaptic inhibitory control in the spinal cord dorsal horn.Ann N Y Acad Sci. 2013 Mar;1279:90-6. doi: 10.1111/nyas.12056. Ann N Y Acad Sci. 2013. PMID: 23531006 Free PMC article. Review.
Cited by
-
Chloride regulation in the pain pathway.Brain Res Rev. 2009 Apr;60(1):149-70. doi: 10.1016/j.brainresrev.2008.12.015. Epub 2008 Dec 31. Brain Res Rev. 2009. PMID: 19167425 Free PMC article. Review.
-
Are all spinal segments equal: intrinsic membrane properties of superficial dorsal horn neurons in the developing and mature mouse spinal cord.J Physiol. 2012 May 15;590(10):2409-25. doi: 10.1113/jphysiol.2012.227389. Epub 2012 Feb 20. J Physiol. 2012. PMID: 22351631 Free PMC article.
-
Transcriptional expression of voltage-gated Na⁺ and voltage-independent K⁺ channels in the developing rat superficial dorsal horn.Neuroscience. 2013 Feb 12;231:305-14. doi: 10.1016/j.neuroscience.2012.11.053. Epub 2012 Dec 7. Neuroscience. 2013. PMID: 23219908 Free PMC article.
-
C-fiber activity-dependent maturation of glycinergic inhibition in the spinal dorsal horn of the postnatal rat.Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):12201-6. doi: 10.1073/pnas.1118960109. Epub 2012 Jul 9. Proc Natl Acad Sci U S A. 2012. PMID: 22778407 Free PMC article.
-
Different forms of glycine- and GABA(A)-receptor mediated inhibitory synaptic transmission in mouse superficial and deep dorsal horn neurons.Mol Pain. 2009 Nov 18;5:65. doi: 10.1186/1744-8069-5-65. Mol Pain. 2009. PMID: 19919721 Free PMC article.
References
-
- Akaike N (1996) Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol 65: 251–264. - PubMed
-
- Andrews K, Fitzgerald M (1994) The cutaneous withdrawal reflex in human neonates: sensitization, receptive fields, and the effects of contralateral stimulation. Pain 56: 95–101. - PubMed
-
- Ben Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3: 728–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
