Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy
- PMID: 15152038
- PMCID: PMC6729468
- DOI: 10.1523/JNEUROSCI.0808-04.2004
Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy
Abstract
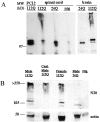
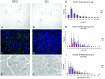
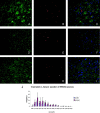
Transgenic models of neurodegenerative disease have proved uniquely powerful for delineating pathways of neuronal dysfunction and cell death. We have developed a transgenic model of the polyglutamine disease spinal and bulbar muscular atrophy (SBMA), an adult-onset, slowly progressive motor neuron disease caused by polyglutamine expansion in the androgen receptor (AR). Mice bearing a human AR with 112 glutamines reproduce many aspects of SBMA, including slowly progressive, gender-specific motor deficits, and neuronal intranuclear inclusions. Despite substantial motor deficits in male AR112Q mice, no motor neuron loss was observed, indicating that neuronal dysfunction, rather than neuronal death, is central to disease. Moreover, reduced levels of unphosphorylated neurofilament heavy chain (NF-H) were observed in motor neurons, suggesting a role for NF-H in SBMA neuronal dysfunction. The elimination of androgens by surgical castration of severely affected, aged 112Q male mice partially restored motor function as well as NF-H levels. These data suggest that hormone-based therapies designed to treat SBMA patients, even with advanced disease, are likely to be effective.
Figures



References
-
- Abel A, Walcott J, Woods J, Duda J, Merry DE (2001) Expression of expanded repeat androgen receptor produces neurologic disease in transgenic mice. Hum Mol Genet 10: 107–116. - PubMed
-
- Chai Y, Koppenhafer SL, Shoesmith SJ, Perez MK, Paulson HL (1999b) Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum Mol Genet 8: 673–682. - PubMed
-
- Clark HB, Burright EN, Yunis WS, Larson S, Wilcox C, Hartman B, Matilla A, Zoghbi HY, Orr HT (1997) Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci 17: 7385–7395. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
