Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors
- PMID: 15152041
- PMCID: PMC6729457
- DOI: 10.1523/JNEUROSCI.5113-03.2004
Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors
Abstract
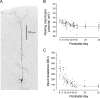
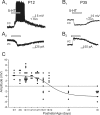
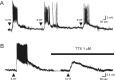
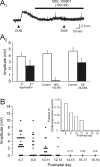
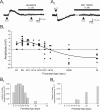
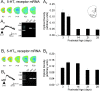
The developing prefrontal cortex receives a dense serotonergic innervation, yet little is known about the actions of serotonin [5-Hydroxytryptamine (5-HT)] in this region during development. Here, we examined the developmental regulation of 5-HT receptors controlling the excitability of pyramidal neurons of this region. Using whole-cell recordings in in vitro brain slices, we identified a dramatic shift in the effects of 5-HT on membrane potential during the postnatal developmental period. In slices derived from young animals [postnatal day (P) 6 to P19], administration of 5-HT elicits a robust depolarization of layer V pyramidal neurons, which gradually shifts to a hyperpolarization commencing during the third postnatal week. This progression is the result of coordinated changes in the function of 5-HT7 and 5-HT2A receptors, which mediate different aspects of the depolarization, and of 5-HT1A receptors, which signal the late developing hyperpolarization. The loss of the 5-HT7 receptor-mediated depolarization and the appearance of the 5-HT1A receptor-mediated hyperpolarization appears to reflect changes in receptor expression. In contrast, the decline in the 5-HT2A receptor depolarization with increasing age was associated with changes in the effectiveness with which these receptors could elicit a membrane depolarization, rather than loss of the receptors per se. Together, these results outline coordinated changes in the serotonergic regulation of cortical excitability at a time of extensive synaptic development and thus suggest a key role for these receptor subtypes in the postnatal development of the prefrontal cortex.
Figures


References
-
- Adham N, Zgombick JM, Bard J, Branchek TA (1998) Functional characterization of the recombinant human 5-hydroxytryptamine7(a) receptor isoform coupled to adenylate cyclase stimulation. J Pharmacol Exp Ther 287: 508–514. - PubMed
-
- Aghajanian GK, Marek GJ (1999) Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res 825: 161–171. - PubMed
-
- Andrade R, Chaput Y (1991) 5-Hydroxytryptamine4-like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J Pharmacol Exp Ther 257: 930–937. - PubMed
-
- Andrade R, Malenka RC, Nicoll RA (1986) A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science 234: 1261–1265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
