Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study
- PMID: 15152042
- PMCID: PMC6729454
- DOI: 10.1523/JNEUROSCI.4203-03.2004
Burst initiation and termination in phasic vasopressin cells of the rat supraoptic nucleus: a combined mathematical, electrical, and calcium fluorescence study
Abstract
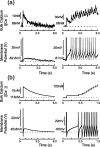
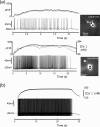
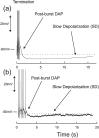
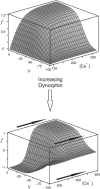
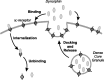
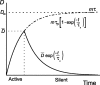
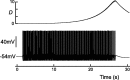
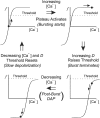
Vasopressin secreting neurons of the rat hypothalamus discharge lengthy, repeating bursts of action potentials in response to physiological stress. Although many electrical currents and calcium-dependent processes have been isolated and analyzed in these cells, their interactions are less well fathomed. In particular, the mechanism of how each burst is triggered, sustained, and terminated is poorly understood. We present a mathematical model for the bursting mechanism, and we support our model with new simultaneous electrical recording and calcium imaging data. We show that bursts can be initiated by spike-dependent calcium influx, and we propose that the resulting elevation of bulk calcium inhibits a persistent potassium current. This inhibition depolarizes the cell above threshold and so triggers regenerative spiking and further calcium influx. We present imaging data to show that bulk calcium reaches a plateau within the first few seconds of the burst, and our model indicates that this plateau occurs when calcium influx is balanced by efflux and uptake into stores. We conjecture that the burst is terminated by a slow, progressive desensitization to calcium of the potassium leak current. Finally, we propose that the opioid dynorphin, which is known to be secreted from the somatodendritic region and has been shown previously to regulate burst length and phasic activity in these cells, is the autocrine messenger for this desensitization.
Figures



Similar articles
-
Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus.J Physiol. 2004 Jun 15;557(Pt 3):949-60. doi: 10.1113/jphysiol.2004.063818. Epub 2004 Apr 23. J Physiol. 2004. PMID: 15107473 Free PMC article.
-
Endogenous activation of supraoptic nucleus kappa-opioid receptors terminates spontaneous phasic bursts in rat magnocellular neurosecretory cells.J Neurophysiol. 2006 May;95(5):3235-44. doi: 10.1152/jn.00062.2006. Epub 2006 Feb 22. J Neurophysiol. 2006. PMID: 16495366
-
Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells.J Neurophysiol. 1984 Mar;51(3):552-66. doi: 10.1152/jn.1984.51.3.552. J Neurophysiol. 1984. PMID: 6321696
-
Intrinsic membrane properties of magnocellular neurosecretory neurons recorded in vitro.Fed Proc. 1986 Aug;45(9):2306-11. Fed Proc. 1986. PMID: 3525230 Review.
-
Electrophysiological distinctions between oxytocin and vasopressin neurons in the supraoptic nucleus.Adv Exp Med Biol. 1998;449:67-77. doi: 10.1007/978-1-4615-4871-3_7. Adv Exp Med Biol. 1998. PMID: 10026787 Review.
Cited by
-
Information coding in vasopressin neurons--the role of asynchronous bistable burst firing.Biosystems. 2013 May;112(2):85-93. doi: 10.1016/j.biosystems.2013.03.010. Epub 2013 Mar 14. Biosystems. 2013. PMID: 23499814 Free PMC article.
-
Phasic spike patterning in rat supraoptic neurones in vivo and in vitro.J Physiol. 2004 Jul 1;558(Pt 1):161-80. doi: 10.1113/jphysiol.2004.063982. Epub 2004 May 14. J Physiol. 2004. PMID: 15146047 Free PMC article.
-
AHP's, HAP's and DAP's: how potassium currents regulate the excitability of rat supraoptic neurones.J Comput Neurosci. 2003 Nov-Dec;15(3):367-89. doi: 10.1023/a:1027424128972. J Comput Neurosci. 2003. PMID: 14618071
-
Quantitative prediction of vasopressin secretion using a computational population model of rat magnocellular neurons.J Comput Neurosci. 2012 Dec;33(3):533-45. doi: 10.1007/s10827-012-0399-3. Epub 2012 Jun 12. J Comput Neurosci. 2012. PMID: 22688885
-
Phasic spiking in vasopressin neurons: How and Why.J Neuroendocrinol. 2021 Nov;33(11):e13042. doi: 10.1111/jne.13042. Epub 2021 Nov 8. J Neuroendocrinol. 2021. PMID: 34748249 Free PMC article. Review.
References
-
- Andrew RD, Dudek FE (1983) Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science 221: 1050–1052. - PubMed
-
- Andrew RD, Dudek FE (1984a) Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol 51: 552–566. - PubMed
-
- Armstrong WE, Sladek CD (1982) Spontaneous “phasic-firing” in supraoptic neurons recorded from hypothalamo-neurohypophysial explants in vitro. Neuroendocrinology 34: 405–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
