Acceleration of visual system development by environmental enrichment
- PMID: 15152044
- PMCID: PMC6729456
- DOI: 10.1523/JNEUROSCI.0845-04.2004
Acceleration of visual system development by environmental enrichment
Abstract
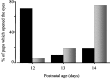
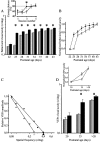
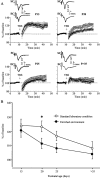
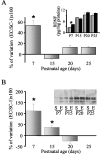
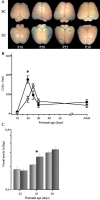
Thus far, the developmental plasticity of the visual system has been studied by altering or reducing visual experience. Here, we investigated whether a complex sensory-motor stimulation, provided by rearing animals in an enriched environment, affects visual system development. We found that raising mice in this condition causes an earlier eye opening, a precocious development of visual acuity, and an accelerated decline of white matter-induced long-term potentiation. These effects are accompanied by a precocious cAMP response element-mediated gene expression and a significant increase of BDNF protein and GAD65/67 expression in enriched pups. In addition, we showed that enriched pups experienced higher levels of licking behavior provided by adult females. Thus, rearing mice from birth in an enriched environment leads to a conspicuous acceleration of visual system development as ascertained at behavioral, electrophysiological, and molecular level.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
