Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties
- PMID: 15152048
- PMCID: PMC6729472
- DOI: 10.1523/JNEUROSCI.5584-03.2004
Infection with an endemic human herpesvirus disrupts critical glial precursor cell properties
Abstract
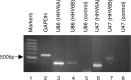
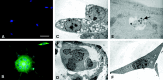
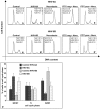
Human herpesvirus 6 (HHV-6), a common resident virus of the human CNS, has been implicated in both acute and chronic inflammatory--demyelinating diseases. Although HHV-6 persists within the human CNS and has been described to infect mature oligodendrocytes, nothing is known about the susceptibility of glial precursors, the ancestors of myelin-producing oligodendrocytes, to viral infection. We show that HHV-6 infects human glial precursor cells in vitro. Active infection was demonstrated by both electron microscopy and expression of viral gene transcripts and proteins, with subsequent formation of cell syncytia. Infection leads to alterations in cell morphology and impairment of cell replication but not increased cell death. Infected cells showed decreased proliferation as measured by bromodeoxyuridine uptake, which was confirmed by blunting of the cell growth rate of infected cells compared with uninfected controls over time. The detailed analysis using novel, fluorescent-labeled HHV-6A or HHV-6B reagents demonstrated strong G1/S phase inhibition in infected precursor cells. Cell cycle arrest in HHV-6-infected cells was associated with a profound decrease in the expression of the glial progenitor cell marker A2B5 and a corresponding increase in the oligodendrocyte differentiation marker GalC. These data demonstrate for the first time that infection of primary human glial precursor cells with a neurologically relevant human herpesvirus causes profound alterations of critical precursor cell properties. In light of recent observations that repair of CNS demyelination is dependent on the generation of mature oligodendrocytes from the glial precursor cell pool, these findings may have broad implications for both the ineffective repair seen in demyelinating diseases and the disruption of normal glial maturation.
Figures




Similar articles
-
Variant-specific tropism of human herpesvirus 6 in human astrocytes.J Virol. 2005 Aug;79(15):9439-48. doi: 10.1128/JVI.79.15.9439-9448.2005. J Virol. 2005. PMID: 16014907 Free PMC article.
-
Infection of murine oligodendroglial precursor cells with Human Herpesvirus 6 (HHV-6)--establishment of a murine in vitro model.J Clin Virol. 2006 Dec;37 Suppl 1:S17-23. doi: 10.1016/S1386-6532(06)70006-3. J Clin Virol. 2006. PMID: 17276361
-
Human herpesvirus 6A infects human embryonic fibroblasts and induces G2/M arrest and cell death.J Med Virol. 2012 Apr;84(4):657-63. doi: 10.1002/jmv.23226. J Med Virol. 2012. PMID: 22337306
-
Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells.J Neurocytol. 2002 Jul-Aug;31(6-7):437-55. doi: 10.1023/a:1025783412651. J Neurocytol. 2002. PMID: 14501215 Review.
-
Roseolovirus-associated encephalitis in immunocompetent and immunocompromised individuals.J Neurovirol. 2017 Feb;23(1):1-19. doi: 10.1007/s13365-016-0473-0. Epub 2016 Aug 18. J Neurovirol. 2017. PMID: 27538995 Free PMC article. Review.
Cited by
-
The role of herpesvirus 6A and 6B in multiple sclerosis and epilepsy.Scand J Immunol. 2020 Dec;92(6):e12984. doi: 10.1111/sji.12984. Epub 2020 Oct 23. Scand J Immunol. 2020. PMID: 33037649 Free PMC article. Review.
-
Human herpesvirus 6A infection in CD46 transgenic mice: viral persistence in the brain and increased production of proinflammatory chemokines via Toll-like receptor 9.J Virol. 2014 May;88(10):5421-36. doi: 10.1128/JVI.03763-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574405 Free PMC article.
-
Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes.J Neurovirol. 2005 Aug;11(4):384-94. doi: 10.1080/13550280591002379. J Neurovirol. 2005. PMID: 16162481 Free PMC article.
-
Classification of HHV-6A and HHV-6B as distinct viruses.Arch Virol. 2014 May;159(5):863-70. doi: 10.1007/s00705-013-1902-5. Epub 2013 Nov 6. Arch Virol. 2014. PMID: 24193951 Free PMC article. Review.
-
Immunolocalization and Distribution of Rubella Antigen in Fatal Congenital Rubella Syndrome.EBioMedicine. 2015 Nov 27;3:86-92. doi: 10.1016/j.ebiom.2015.11.050. eCollection 2016 Jan. EBioMedicine. 2015. PMID: 26870820 Free PMC article.
References
-
- Albright AV, Lavi E, Black JB, Goldberg S, O'Connor MJ, Gonzalez-Scarano F (1998) The effect of human herpesvirus-6 (HHV-6) on cultured human neural cells: oligodendrocytes and microglia. J Neurovirol 4: 486–494. - PubMed
-
- Bitsch A, Kuhlman T, Da Costa C, Bunkowski S, Polak T, Bruck W (2000) Tumoure necrosis factor alpha mRNA expression in early multiple sclerosis lesions: correlation with demyelinating activity and oligodendrocyte pathology. Glia 29: 366–375. - PubMed
-
- Blakemore WF, Keirstead HS (1999) The origin of remyelinating cells in the central nervous system. J Neuroimmunol 98: 69–76. - PubMed
-
- Blumberg BM, Mock DJ, Powers JM, Ito M, Assouline JG, Baker JV, Chen B, Goodman AD (2000) The HHV6 paradox: ubiquitous commensal or insidious pathogen? A two-step in situ PCR approach. J Clin Virol 16: 159–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
