Cholecystokinin blockade alters the systemic immune response in rats with acute pancreatitis
- PMID: 15154913
- PMCID: PMC2517463
- DOI: 10.1111/j.0959-9673.2004.00372.x
Cholecystokinin blockade alters the systemic immune response in rats with acute pancreatitis
Abstract
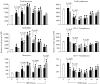
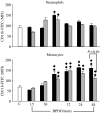
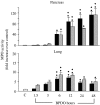
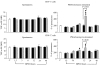
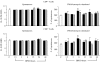
Acute pancreatitis (AP) is characterized by initial pancreatic injury resulting from the activation of digestive enzymes and, later, widespread inflammation to distant organs. The aim of this study was to study whether the time-course of inflammatory events during AP induced by bile-pancreatic duct obstruction (BPDO) varies after lowering the acinar enzyme content by L364,718 (0.1 mg/kg/day) administration over 7 days before inducing AP. Flow cytometric immunophenotyping was used to analyse the following at different AP stages: distribution of major circulating leucocyte subsets, activation state of circulating neutrophils and monocytes as reflected by CD11b expression and tumour necrosis factor-alpha (TNF-alpha) production and the contribution of T-cell-derived pro-(TNF-alpha) and anti-(IL-10) inflammatory mediators. TNF-alpha plasma levels and neutrophil infiltration in pancreas and lung were also measured. At early BPDO times, L364,718 treatment partially inhibited leukocytosis and increase in peripheral blood neutrophils and monocytes as well as TNF-alpha expression by monocytes. However, from 6 h onwards after BPDO, L364,718 treatment was unable to prevent either pancreatic and lung neutrophil infiltration or the release of TNF-alpha from activated monocytes. By its action on circulating lymphocytes, L364,718 treatment enhanced the severity of the inflammatory response induced by BPDO. Peripheral blood lymphocytes were recruited from earlier BPDO times, and 12 h after BPDO, T cells displayed a significantly higher reserve of TNF-alpha able to be released under stimulation but lower functional reserve of interleukin-10 (IL-10) than observed in untreated rats. It is concluded that lowering the acinar enzyme content through L364,718 treatment prevents earlier systemic immune events in BPDO-induced AP. However, at the point of maximal injury, the inflammatory response became pronounced, largely due to the role played by activated T lymphocytes.
Figures
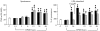
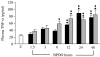
References
-
- Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J. Pathol. 2000a;190:117–125. - PubMed
-
- De Beaux AC, Ross JA, Maingay JP, Fearon KCH, Carter DC. Proinflammatory cytokine release by peripheral blood mononuclear cells from patients with acute pancreatitis. Br. J. Surg. 1996;83:1071–1075. - PubMed
-
- De Dios I, Perez M, De la Mano A, et al. Contribution of circulating leukocytes to cytokine production in pancreatic duct obstruction-induced acute pancreatitis in rats. Cytokine. 2002;20:295–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

