Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells
- PMID: 15155186
- PMCID: PMC415601
- DOI: 10.1128/AAC.48.6.1968-1973.2004
Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells
Abstract
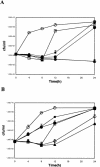
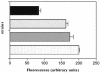
Epigallocatechin-gallate (EGCg), the major catechin present in green tea extracts, has been shown to have several antibacterial activities, limiting bacterial growth and invasion and acting in synergy with beta-lactam antibiotics. In this article, we report that EGCg at doses half and below its calculated MIC of 100 microg/ml, is able to reverse tetracycline resistance in staphylococcal isolates expressing the specific efflux pump Tet(K) and appears to improve the MICs of tetracycline for susceptible staphylococcal isolates as well. The visible effect of EGCg is an increased accumulation of tetracycline inside bacterial cells. This effect is likely due to the inhibition of pump activity, and it is evident not only for Tet(K) pumps but also for efflux pumps of a different class [Tet(B)]. In summary, our data indicate that the observed dramatic enhancement by EGCg of tetracycline activity for resistant staphylococcal isolates is caused by impairment of tetracycline efflux pump activity and increased intracellular retention of the drug, suggesting a possible use of EGCg as an adjuvant in antibacterial therapy.
Figures

Similar articles
-
Synergistic interactions of epigallocatechin gallate and oxytetracycline against various drug resistant Staphylococcus aureus strains in vitro.Phytomedicine. 2013 Mar 15;20(5):432-5. doi: 10.1016/j.phymed.2012.12.010. Epub 2013 Feb 26. Phytomedicine. 2013. PMID: 23485046
-
Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus.Antimicrob Agents Chemother. 2001 Jun;45(6):1737-42. doi: 10.1128/AAC.45.6.1737-1742.2001. Antimicrob Agents Chemother. 2001. PMID: 11353619 Free PMC article.
-
Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates.Antimicrob Agents Chemother. 2005 Oct;49(10):4339-43. doi: 10.1128/AAC.49.10.4339-4343.2005. Antimicrob Agents Chemother. 2005. PMID: 16189116 Free PMC article.
-
The inhibition the Tet(K) efflux pump of tetracycline resistant Staphylococcus epidermidis by essential oils from three Salvia species.Lett Appl Microbiol. 2015 Jul;61(1):58-62. doi: 10.1111/lam.12424. Epub 2015 May 1. Lett Appl Microbiol. 2015. PMID: 25846244
-
The tetracycline resistome.Cell Mol Life Sci. 2010 Feb;67(3):419-31. doi: 10.1007/s00018-009-0172-6. Epub 2009 Oct 28. Cell Mol Life Sci. 2010. PMID: 19862477 Free PMC article. Review.
Cited by
-
Applications of Catechins in the Treatment of Bacterial Infections.Pathogens. 2021 May 1;10(5):546. doi: 10.3390/pathogens10050546. Pathogens. 2021. PMID: 34062722 Free PMC article. Review.
-
A Putative Efflux Transporter of the ABC Family, YbhFSR, in Escherichia coli Functions in Tetracycline Efflux and Na+(Li+)/H+ Transport.Front Microbiol. 2020 Apr 23;11:556. doi: 10.3389/fmicb.2020.00556. eCollection 2020. Front Microbiol. 2020. PMID: 32390957 Free PMC article.
-
Efflux pump inhibitors for bacterial pathogens: From bench to bedside.Indian J Med Res. 2019 Feb;149(2):129-145. doi: 10.4103/ijmr.IJMR_2079_17. Indian J Med Res. 2019. PMID: 31219077 Free PMC article. Review.
-
Identification of Human Kinin-Forming Enzyme Inhibitors from Medicinal Herbs.Molecules. 2021 Jul 7;26(14):4126. doi: 10.3390/molecules26144126. Molecules. 2021. PMID: 34299400 Free PMC article.
-
Phytochemicals in Drug Discovery-A Confluence of Tradition and Innovation.Int J Mol Sci. 2024 Aug 13;25(16):8792. doi: 10.3390/ijms25168792. Int J Mol Sci. 2024. PMID: 39201478 Free PMC article. Review.
References
-
- Blanco, A. R., S. La Terra Mule, G. Babini, S. Garbisa, V. Enea, and D. Rusciano. 2003. (−)Epigallocatechin-3-gallate inhibits gelatinase activity of some bacterial isolates from ocular infection, and limits their invasion through gelatine. Biochim. Biophys. Acta 1620:273-281. - PubMed
-
- Chopra, I., P. M. Hawkey, and M. Hinton. 1992. Tetracyclines: molecular and clinical aspects. J. Antimicrob. Chemother. 29:245-277. - PubMed
-
- Cohen, M. L. 1992. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 257:1050-1055. - PubMed
-
- Higdon, J. V., and B. Frei. 2003. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 43:89-143. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

