Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration
- PMID: 15155217
- PMCID: PMC415618
- DOI: 10.1128/AAC.48.6.2166-2172.2004
Pharmacokinetics and safety of intravenous voriconazole in children after single- or multiple-dose administration
Abstract
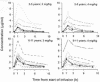
We conducted a multicenter study of the safety, tolerability, and plasma pharmacokinetics of the parenteral formulation of voriconazole in immunocompromised pediatric patients (2 to 11 years old). Single doses of 3 or 4 mg/kg of body weight were administered to six and five children, respectively. In the multiple-dose study, 28 patients received loading doses of 6 mg/kg every 12 h on day 1, followed by 3 mg/kg every 12 h on day 2 to day 4 and 4 mg/kg every 12 h on day 4 to day 8. Standard population pharmacokinetic approaches and generalized additive modeling were used to construct the structural pharmacokinetic and covariate models used in this analysis. In contrast to that in adult healthy volunteers, elimination of voriconazole was linear in children following doses of 3 and 4 mg/kg every 12 h. Body weight was more influential than age in accounting for the observed variability in voriconazole pharmacokinetics. Elimination capacity correlated with the CYP2C19 genotype. Exposures were similar at 4 mg/kg every 12 h in children (median area under the concentration-time curve (AUC), 14,227 ng. h/ml) and 3 mg/kg in adults (median AUC, 13,855 ng. h/ml). Visual disturbances occurred in 5 (12.8%) of the 39 patients and were the only drug-related adverse events that occurred more than once. No withdrawals from the study were related to voriconazole. We conclude that pediatric patients have a higher capacity for elimination of voriconazole per kilogram of body weight than do adult healthy volunteers and that dosages of 4 mg/kg may be required in children to achieve exposures consistent with those in adults following dosages of 3 mg/kg.
Figures

References
-
- Ally, R., D. Schurmann, W. Kreisel, G. Carosi, K. Aguirrebengoa, B. Dupont, M. Hodges, P. Troke, A. J. Romero, and the Esophageal Candidiasis Study Group. 2001. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin. Infect. Dis. 33:1447-1454. - PubMed
-
- Beal, S. L., L. B. Sheiner, et al. (ed.). 1992. NONMEM users guides. University of California at San Francisco, San Francisco, Calif.
-
- Chiou, C. C., A. H. Groll, and T. J. Walsh. 2000. New drugs and novel targets for treatment of invasive fungal infections in patients with cancer. Oncologist 5:120-135. - PubMed
-
- Denning, D. W., P. Ribaud, N. Milpied, D. Caillot, R. Herbrecht, E. Thiel, A. Haas, M. Ruhnke, and H. Lode. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin. Infect. Dis. 34:563-571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

