Structure and association of human lactoferrin peptides with Escherichia coli lipopolysaccharide
- PMID: 15155221
- PMCID: PMC415569
- DOI: 10.1128/AAC.48.6.2190-2198.2004
Structure and association of human lactoferrin peptides with Escherichia coli lipopolysaccharide
Abstract
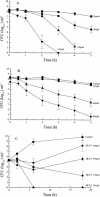
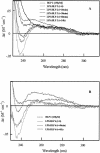
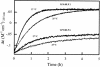
An 11-amino-acid amphipathic synthetic peptide homologous to a helical region on helix 1 of human lactoferrin HLP-2 exhibited bactericidal activity against Escherichia coli serotype O111, whereas an analogue synthesized with Pro substituted for Met, HLP-6, had greatly reduced antimicrobial activity. The bactericidal activity of HLP-2 was 10-fold greater than that of HLP-6 in both buffer and growth medium by time-kill assays. These assays also showed a pronounced lag phase that was both concentration and time dependent and that was far greater for HLP-2 than for HLP-6. Both peptides, however, were shown to be equally efficient in destabilizing the outer membrane when the hydrophobic probe 1-N-phenylnaphthylamine was used and to have the same lipopolysaccharide (LPS) binding affinity, as shown by polymyxin B displacement. Circular dichroism (CD) spectroscopy was used to study the structure and the organization of the peptides in solution and upon interaction with E. coli LPS. In the presence of LPS, HLP-2 and HLP-6 were found to bind and adopt a beta-strand conformation rather than an alpha-helix, as shown by nonimmobilized ligand interaction assay-CD spectroscopy. Furthermore, this assay was used to show that there is a time-dependent association of peptide that results in an ordered formation of peptide aggregates. The rate of interpeptide association was far greater in HLP-2 LPS than in HLP-6 LPS, which was consistent with the lag phase observed on the killing curves. These results allow us to propose a mechanism by which HLP-2 folds and self-assembles at the outer membrane surface before exerting its activity.
Figures



Similar articles
-
Structure-function relationship of antibacterial synthetic peptides homologous to a helical surface region on human lactoferrin against Escherichia coli serotype O111.Infect Immun. 1998 Jun;66(6):2434-40. doi: 10.1128/IAI.66.6.2434-2440.1998. Infect Immun. 1998. PMID: 9596699 Free PMC article.
-
A helical region on human lactoferrin. Its role in antibacterial pathogenesis.Adv Exp Med Biol. 1998;443:215-20. Adv Exp Med Biol. 1998. PMID: 9781361
-
Variation in antimicrobial activity of lactoferricin-derived peptides explained by structure modelling.FEMS Microbiol Lett. 2004 Sep 1;238(1):221-6. doi: 10.1016/j.femsle.2004.07.038. FEMS Microbiol Lett. 2004. PMID: 15336425
-
A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides.J Biol Chem. 2005 Mar 18;280(11):10378-87. doi: 10.1074/jbc.M412865200. Epub 2005 Jan 4. J Biol Chem. 2005. PMID: 15632151
-
Peptides with dual mode of action: Killing bacteria and preventing endotoxin-induced sepsis.Biochim Biophys Acta. 2016 May;1858(5):971-9. doi: 10.1016/j.bbamem.2016.01.011. Epub 2016 Jan 20. Biochim Biophys Acta. 2016. PMID: 26801369 Review.
Cited by
-
QSAR modeling: where have you been? Where are you going to?J Med Chem. 2014 Jun 26;57(12):4977-5010. doi: 10.1021/jm4004285. Epub 2014 Jan 6. J Med Chem. 2014. PMID: 24351051 Free PMC article.
-
Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment.Int J Oral Sci. 2017 Nov 10;9(11):e1. doi: 10.1038/ijos.2017.35. Int J Oral Sci. 2017. PMID: 29125139 Free PMC article. Review.
-
Lactoferrins in Their Interactions with Molecular Targets: A Structure-Based Overview.Pharmaceuticals (Basel). 2024 Mar 20;17(3):398. doi: 10.3390/ph17030398. Pharmaceuticals (Basel). 2024. PMID: 38543184 Free PMC article. Review.
-
Human amylin is a potent antimicrobial peptide that exhibits antimicrobial synergism with the amyloid beta protein.Alzheimers Dement. 2025 Aug;21(8):e70490. doi: 10.1002/alz.70490. Alzheimers Dement. 2025. PMID: 40731182 Free PMC article.
-
Structure, dynamics and kinetics of two-component Lantibiotic Lichenicidin.PLoS One. 2017 Jun 27;12(6):e0179962. doi: 10.1371/journal.pone.0179962. eCollection 2017. PLoS One. 2017. PMID: 28654661 Free PMC article.
References
-
- Bello, T., H. R. Bello, and E. Granados. 1982. Conformation and aggregation of melittin: dependence on pH and concentration. Biochemistry 21:461-465. - PubMed
-
- Bevins, C. 1994. Antimicrobial peptides as agents of mucosal immunity. Ciba Found. Symp. 186:250-269. - PubMed
-
- Boman, H. G. 1995. Peptide antibiotics and their role in innate immmunity. Annu. Rev. Immunol. 13:61-92. - PubMed
-
- Chapple, D. S., D. J. Mason, C. L. Joannou, E. W. Odell, V. Gant, and R. W. Evans. 1998. Structure-function relationship of antibacterial synthetic peptides homologous to a helical surface region on human lactoferrin against Escherschia coli serotype O111. Infect. Immun. 66:2434-2440. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

