Phosphorothioate di- and trinucleotides as a novel class of anti-hepatitis B virus agents
- PMID: 15155222
- PMCID: PMC415564
- DOI: 10.1128/AAC.48.6.2199-2205.2004
Phosphorothioate di- and trinucleotides as a novel class of anti-hepatitis B virus agents
Abstract
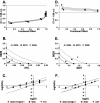
Several nucleoside analogs are under clinical development for use against hepatitis B virus (HBV). Lamivudine (3TC), a nucleoside analog, and adefovir dipivoxil (ADV), an acyclonucleotide analog, are clinically approved. However, long-term treatment can induce viral resistance, and following the cessation of therapy, viral rebound is frequently observed. There continues to be a need for new antiviral agents with novel mechanisms of action. A library of more than 600 di- and trinucleotide compounds synthesized by parallel synthesis using a combinatorial strategy was screened for potential inhibitors of HBV replication using the chronically HBV-producing cell line 2.2.15. Through an iterative process of synthesis, lead optimization, and screening, three analogs were identified as potent inhibitors of HBV replication: dinucleotides ORI-7246 (drug concentration at which a 10-fold reduction of HBV DNA was observed [EC(90)], 1.4 microM) and ORI-9020 (EC(90), 1.2 microM) and trinucleotide ORI-7170 (EC(90), 7.2 microM). These analogs inhibited the replication of both strands of HBV DNA. No suppression of HBV protein synthesis or intracellular core particle formation by these analogs was observed. No inhibition of HBV DNA strand elongation by the analogs or their 5'-triphosphate versions was apparent in in vitro polymerase assays. Although the exact mechanism of action is not yet identified, present data are consistent with an inhibition of the HBV reverse transcriptase-directed priming step prior to elongation of the first viral DNA strand. In transient-transfection assays, these analogs inhibited the replication of 3TC-resistant HBV. Synergistic interactions in combination treatments between the analogs and either 3TC or ADV were observed. These compounds represent a novel class of anti-HBV molecules and warrant further investigation as potential therapeutic agents.
Figures
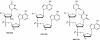

Similar articles
-
In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir.Antivir Ther. 2004 Jun;9(3):353-63. Antivir Ther. 2004. PMID: 15259898
-
Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir.Hepatology. 2005 Jun;41(6):1391-8. doi: 10.1002/hep.20723. Hepatology. 2005. PMID: 15915463
-
In vitro characterization of the anti-hepatitis B virus activity and cross-resistance profile of 2',3'-dideoxy-3'-fluoroguanosine.Antimicrob Agents Chemother. 2006 Mar;50(3):955-61. doi: 10.1128/AAC.50.3.955-961.2006. Antimicrob Agents Chemother. 2006. PMID: 16495257 Free PMC article.
-
Assessing hepatitis B virus resistance in vitro and molecular mechanisms of nucleoside resistance.Semin Liver Dis. 2002;22 Suppl 1:23-31. doi: 10.1055/s-2002-35697. Semin Liver Dis. 2002. PMID: 12447726 Review.
-
Resistance of hepatitis B virus to antiviral drugs: current aspects and directions for future investigation.Antivir Chem Chemother. 2001 Jan;12(1):1-35. doi: 10.1177/095632020101200101. Antivir Chem Chemother. 2001. PMID: 11437320 Review.
Cited by
-
Small-molecule effectors of hepatitis B virus capsid assembly give insight into virus life cycle.J Virol. 2008 Oct;82(20):10262-70. doi: 10.1128/JVI.01360-08. Epub 2008 Aug 6. J Virol. 2008. PMID: 18684823 Free PMC article.
-
Alkoxyalkyl esters of 9-(s)-(3-hydroxy-2-phosphonomethoxypropyl) adenine are potent and selective inhibitors of hepatitis B virus (HBV) replication in vitro and in HBV transgenic mice in vivo.Antimicrob Agents Chemother. 2009 Jul;53(7):2865-70. doi: 10.1128/AAC.00114-09. Epub 2009 Apr 27. Antimicrob Agents Chemother. 2009. PMID: 19398648 Free PMC article.
-
In vitro inhibition effects of hepatitis B virus by dandelion and taraxasterol.Infect Agent Cancer. 2020 Jul 6;15:44. doi: 10.1186/s13027-020-00309-4. eCollection 2020. Infect Agent Cancer. 2020. PMID: 32647534 Free PMC article.
-
SB 9200, a novel agonist of innate immunity, shows potent antiviral activity against resistant HCV variants.J Med Virol. 2017 Sep;89(9):1620-1628. doi: 10.1002/jmv.24809. Epub 2017 May 23. J Med Virol. 2017. PMID: 28303593 Free PMC article.
-
Neurobiological applications of small molecule screening.Chem Rev. 2008 May;108(5):1774-86. doi: 10.1021/cr0782372. Epub 2008 May 1. Chem Rev. 2008. PMID: 18447397 Free PMC article. Review. No abstract available.
References
-
- Allen, M. I., M. Deslauriers, C. W. Andrews, G. A. Tipples, K. A. Walters, D. L. Tyrrell, N. Brown, L. D. Condreay, et al. 1998. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology 27:1670-1677. - PubMed
-
- Balfour, H. H., Jr. 1999. Antiviral drugs. N. Engl. J. Med. 340:1255-1268. - PubMed
-
- Belen'kii, M. S., and R. Schinazi. 1994. A method for the analysis of combination therapies with statistical analysis. Antivir. Res. 25:11-18.
-
- Bryant, M. L., E. G. Bridges, L. Placidi, A. Faraj, A. G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J. L. Imbach, B. Hernandez, A. Juodawlkis, B. Tennant, B. Korba, P. Cote, P. Marion, E. Cretton-Scott, R. F. Schinazi, and J. P. Sommadossi. 2001. Antiviral l-nucleosides specific for hepatitis B virus infection. Antimicrob. Agents Chemother. 45:229-235. - PMC - PubMed
-
- Chauret, N., A. Gauthier, J. Martin, and D. A. Nicoll-Griffith. 1997. In vitro comparison of cytochrome P450-mediated metabolic activities in human, dog, cat and horse. Drug Metab. Dispos. 25:1130-1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

