Potential new anti-human immunodeficiency virus type 1 compounds depress virus replication in cultured human macrophages
- PMID: 15155246
- PMCID: PMC415615
- DOI: 10.1128/AAC.48.6.2325-2330.2004
Potential new anti-human immunodeficiency virus type 1 compounds depress virus replication in cultured human macrophages
Abstract
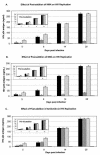
We report that the amiloride analogues 5-(N,N-hexamethylene)amiloride and 5-(N,N-dimethyl)amiloride inhibit, at micromolar concentrations, the replication of human immunodeficiency virus type 1 (HIV-1) in cultured human blood monocyte-derived macrophages. These compounds also inhibit the in vitro activities of the HIV-1 Vpu protein and might represent lead compounds for a new class of anti-HIV-1 drugs.
Figures



Similar articles
-
Amiloride derivatives block ion channel activity and enhancement of virus-like particle budding caused by HIV-1 protein Vpu.Eur Biophys J. 2002 Mar;31(1):26-35. doi: 10.1007/s002490100177. Eur Biophys J. 2002. PMID: 12046895
-
Antiviral efficacy of the novel compound BIT225 against HIV-1 release from human macrophages.Antimicrob Agents Chemother. 2010 Feb;54(2):835-45. doi: 10.1128/AAC.01308-09. Epub 2009 Dec 7. Antimicrob Agents Chemother. 2010. PMID: 19995924 Free PMC article.
-
Lack of effect of recombinant human growth hormone on the in vitro activities of antiretroviral drugs against human immunodeficiency virus type 1.Antimicrob Agents Chemother. 2004 Jun;48(6):2337-40. doi: 10.1128/AAC.48.6.2337-2340.2004. Antimicrob Agents Chemother. 2004. PMID: 15155249 Free PMC article.
-
Inhibition of HIV-1 replication by targeting the Rev protein.Leukemia. 1997 Apr;11 Suppl 3:134-7. Leukemia. 1997. PMID: 9209321 Review.
-
Multiple effects of interferon on the replication of human immunodeficiency virus type 1.Antiviral Res. 1994 Jul;24(2-3):205-19. doi: 10.1016/0166-3542(94)90068-x. Antiviral Res. 1994. PMID: 7526792 Review.
Cited by
-
Viral targets of acylguanidines.Drug Discov Today. 2012 Sep;17(17-18):1039-43. doi: 10.1016/j.drudis.2012.05.002. Epub 2012 May 8. Drug Discov Today. 2012. PMID: 22580299 Free PMC article. Review.
-
Novel Compound Inhibitors of HIV-1NL4-3 Vpu.Viruses. 2022 Apr 15;14(4):817. doi: 10.3390/v14040817. Viruses. 2022. PMID: 35458546 Free PMC article.
-
Requirement of a functional ion channel for Sindbis virus glycoprotein transport, CPV-II formation, and efficient virus budding.PLoS Pathog. 2022 Oct 3;18(10):e1010892. doi: 10.1371/journal.ppat.1010892. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36191050 Free PMC article.
-
Modulation of influenza virus replication by alteration of sodium ion transport and protein kinase C activity.Antiviral Res. 2008 Nov;80(2):124-34. doi: 10.1016/j.antiviral.2008.05.008. Epub 2008 Jun 13. Antiviral Res. 2008. PMID: 18585796 Free PMC article.
-
The HIV-1 Vpu viroporin inhibitor BIT225 does not affect Vpu-mediated tetherin antagonism.PLoS One. 2011;6(11):e27660. doi: 10.1371/journal.pone.0027660. Epub 2011 Nov 14. PLoS One. 2011. PMID: 22110710 Free PMC article.
References
-
- Balliet, J. W., D. L. Kolson, G. Eiger, F. M. Kim, K. A. McGann, A. Srinivasan, and R. Collman. 1994. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology 200:623-631. - PubMed
-
- Carr, J. M., H. Hocking, P. Li, and C. J. Burrell. 1999. Rapid and efficient cell to cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265:319-329. - PubMed
-
- Du, B., A. Wolf, S. Lee, and E. Terwilliger. 1993. Changes in the host range and growth potential of an HIV-1 clone are conferred by the vpu gene. Virology 195:260-264. - PubMed
-
- Ewart, G. D., K. Mills, G. B. Cox, and P. W. Gage. 2002. Amiloride derivatives block ion channel activity and enhancement of virus-like particle budding caused by HIV-1 protein Vpu. Eur. Biophys. J. 31:26-35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

