Molecular mechanisms of vasoselectivity of the 1,4-dihydropyridine lercanidipine
- PMID: 15155536
- PMCID: PMC1574954
- DOI: 10.1038/sj.bjp.0705786
Molecular mechanisms of vasoselectivity of the 1,4-dihydropyridine lercanidipine
Abstract
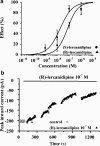
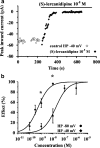
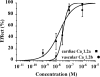
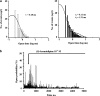
The effects of (S)- and (R)-lercanidipine on CHO cells stably expressing the cardiac (Ca(v)1.2a) or vascular (Ca(v)1.2b) splice variant of the L-type calcium channel pore subunit were studied, using whole-cell and single-channel patch-clamp measurements. Lercanidipine block of Ca(v)1.2b current was enantioselective. (S)-lercanidipine was 4.1-fold more potent. Experiments using acidic solutions (pH 6.8) revealed a 6.4-fold enhanced inhibitory effect of (S)-lercanidipine compared with physiological conditions (pH 7.4) indicating that the charged form mediates inhibition. At depolarised holding potential (-40 mV), (S)-lercanidipine exhibited a 35-fold greater potency, compared with standard conditions (-80 mV). A comparison of the concentration-dependent inhibition of Ca(v)1.2a with Ca(v)1.2b subunit currents by (S)-lercanidipine revealed only a 1.8-fold difference in IC(50), but the slope of the dose-response curve was much steeper (n(H)=2.3) with Ca(v)1.2a, compared with Ca(v)1.2b (n(H)=0.8). This indicates overlap between agonistic and antagonistic effects, predominant with the cardiac Ca(v)1.2a subunit. This idea is supported by transient stimulatory effects, and a slight leftward shift of the IV curves. These effects were more prominent for Ca(v)1.2a than for Ca(v)1.2b. Single-channel experiments confirmed typical features of calcium channel agonists such as prolonged channel openings, a component of lengthened openings, and an enhanced open probability in the presence of (S)-lercanidipine. Again, these findings were concentration-dependent and more pronounced for Ca(v)1.2a than for Ca(v)1.2b. Our data indicate a splice-variant predominant agonism as a new mechanism contributing to the vasoselectivity of lercanidipine, along with marked voltage-dependence of action.
Figures





Similar articles
-
Pharmacological in vitro studies of the new 1,4-dihydropyridine calcium antagonist lercanidipine.Arzneimittelforschung. 1996 Jan;46(1):15-24. Arzneimittelforschung. 1996. PMID: 8821512
-
Kinetics and Gbetagamma modulation of Ca(v)2.2 channels with different auxiliary beta subunits.Pflugers Arch. 2002 May;444(1-2):263-75. doi: 10.1007/s00424-002-0803-3. Epub 2002 Mar 9. Pflugers Arch. 2002. PMID: 11976940
-
Studies on Ca channels in intact cardiac cells: voltage-dependent effects and cooperative interactions of dihydropyridine enantiomers.Mol Pharmacol. 1986 Dec;30(6):571-84. Mol Pharmacol. 1986. PMID: 2431263
-
Voltage-dependent calcium channels.Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review.
-
L-type Ca2+ channels of the embryonic mouse heart.Eur J Pharmacol. 2002 Jul 5;447(2-3):279-84. doi: 10.1016/s0014-2999(02)01850-2. Eur J Pharmacol. 2002. PMID: 12151019 Review.
Cited by
-
Lercanidipine in hypertension.Vasc Health Risk Manag. 2005;1(3):173-82. Vasc Health Risk Manag. 2005. PMID: 17319103 Free PMC article. Review.
-
Screening of FDA-Approved Drugs Using a MERS-CoV Clinical Isolate from South Korea Identifies Potential Therapeutic Options for COVID-19.Viruses. 2021 Apr 9;13(4):651. doi: 10.3390/v13040651. Viruses. 2021. PMID: 33918958 Free PMC article.
-
New Insights into the Nephroprotective Potential of Lercanidipine.Int J Mol Sci. 2023 Sep 13;24(18):14048. doi: 10.3390/ijms241814048. Int J Mol Sci. 2023. PMID: 37762350 Free PMC article. Review.
References
-
- ANGELICO P., GUARNERI A., LEONARDI A., TESTA R. Vascular-selective effect of lercanidipine and other 1,4-dihydropyridines in isolated rabbit tissues. J. Pharm. Pharmacol. 1999;51:709–714. - PubMed
-
- BAINDUR N., RUTLEDGE A., TRIGGLE D.J. A homologous series of permanently charged 1,4-dihydropyridines: novel probes designed to localize drug binding sites on ion channels. J. Med. Chem. 1993;36:3743–3745. - PubMed
-
- BANGALORE R., BAINDUR N., RUTLEDGE A., TRIGGLE D.J., KASS R.S. L-type calcium channels: asymmetrical intramembrane binding domain revealed by variable length, permanently charged 1,4-dihydropyridines. Mol. Pharmacol. 1994;46:660–666. - PubMed
-
- BECHEM M., HOFFMANN H. The molecular mode of action of the Ca agonist (−) Bay K 8644 on the cardiac Ca channel. Pflügers Arch. 1993;424:343–353. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

