The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments
- PMID: 15155583
- PMCID: PMC415645
- DOI: 10.1101/gad.300104
The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments
Abstract
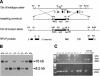
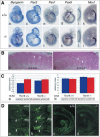
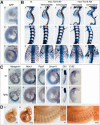
The compartmentalization of somites along their anterior-posterior (AP) axis is pivotal to the segmental organization of the vertebrate axial skeleton and the peripheral nervous system. Anterior and posterior somite halves contribute to different vertebral elements. They are also characterized by different proliferation rates and properties with respect to neural crest cell migration and spinal nerve passage. AP-somite polarity is generated in the anterior presomitic mesoderm by Mesp2 and Delta/Notch signaling. Here, we demonstrate that maintenance of AP-somite polarity is mediated by the T-box transcription factor Tbx18. Mice deficient for Tbx18 show expansion of pedicles with transverse processes and proximal ribs, elements derived from the posterior lateral sclerotome. AP-somite polarity is established in Tbx18 mutant embryos but is not maintained. During somite maturation, posterior somite compartments expand most likely because of posterior cells invading the anterior somite half. In the anterior lateral sclerotome, Tbx18 acts as an antiapoptotic factor. Ectopic expression experiments suggest that Tbx18 can promote anterior at the expense of posterior somite compartments. In summary, Tbx18 appears to act downstream of Mesp2 and Delta/Notch signaling to maintain the separation of anterior and posterior somite compartments.
Figures


References
-
- Aoyama H. and Asamoto, K. 2000. The developmental fate of the rostral/caudal half of a somite for vertebra and rib formation: Experimental confirmation of the resegmentation theory using chick-quail chimeras. Mech. Dev. 99: 71–82. - PubMed
-
- Barrantes del Barco I., Elia, A.J., Wünsch, K., Hrabe De Angelis, M., Mak, T.W., Rossant, R., Conlon, R.A., Gossler, A., and Pompa, J-L. 1999. Interaction between L-fringe and Notch signalling in the regulation of boundary formation and posterior identity in the presomitic mesoderm of the mouse. Curr. Biol. 9: 470–480. - PubMed
-
- Beckers J., Caron, A., Hrabe de Angelis, M., Hans, S., Campos-Ortega, J.A., and Gossler, A. 2000. Distinct regulatory elements direct Delta1 expression in the nervous system and paraxial mesoderm of transgenic mice. Mech. Dev. 95: 23–34. - PubMed
-
- Bettenhausen B., de Angelis, M.H., Simon, D., Guénet, J.-L., and Gossler, A. 1995. Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila Delta. Development 121: 2407–2418. - PubMed
-
- Biben C., Stanley, E., Fabri, L., Kotecha, S., Rhinn, M., Drink-water, C., Lah, M., Wang, C.C., Nash, A., Hilton, D., et al. 1998. Murine cerberus homologue mCer-1: A candidate anterior patterning molecule. Dev. Biol. 194: 135–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
