Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle
- PMID: 15155609
- PMCID: PMC415675
- DOI: 10.1128/IAI.72.6.3089-3096.2004
Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle
Figures
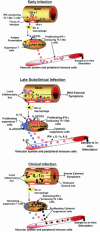
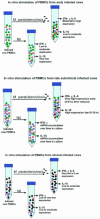
Similar articles
-
Secreted antigens of Mycobacterium avium subspecies paratuberculosis as prominent immune targets.Vet Microbiol. 2006 May 31;114(3-4):337-44. doi: 10.1016/j.vetmic.2005.12.005. Epub 2006 Jan 18. Vet Microbiol. 2006. PMID: 16413703
-
Host responses to persistent Mycobacterium avium subspecies paratuberculosis infection in surgically isolated bovine ileal segments.Clin Vaccine Immunol. 2013 Feb;20(2):156-65. doi: 10.1128/CVI.00496-12. Epub 2012 Dec 5. Clin Vaccine Immunol. 2013. PMID: 23221000 Free PMC article.
-
Mycobacterium avium subspecies paratuberculosis infection of cattle does not diminish peripheral blood-derived macrophage mycobactericidal activity.Immunol Lett. 2006 Sep 15;107(1):76-9. doi: 10.1016/j.imlet.2006.06.004. Epub 2006 Jul 7. Immunol Lett. 2006. PMID: 16884783
-
[Pathogenesis and immune reactions of paratuberculosis].Dtsch Tierarztl Wochenschr. 2002 Dec;109(12):507-9. Dtsch Tierarztl Wochenschr. 2002. PMID: 12596563 Review. German.
-
Mycobacterium paratuberculosis and the bovine immune system.Anim Health Res Rev. 2001 Dec;2(2):141-61. Anim Health Res Rev. 2001. PMID: 11831436 Review.
Cited by
-
Bovine Neutrophils Release Extracellular Traps and Cooperate With Macrophages in Mycobacterium avium subsp. paratuberculosis clearance In Vitro.Front Immunol. 2021 Mar 17;12:645304. doi: 10.3389/fimmu.2021.645304. eCollection 2021. Front Immunol. 2021. PMID: 33815401 Free PMC article.
-
Pan-genomic analysis of bovine monocyte-derived macrophage gene expression in response to in vitro infection with Mycobacterium avium subspecies paratuberculosis.Vet Res. 2012 Mar 28;43(1):25. doi: 10.1186/1297-9716-43-25. Vet Res. 2012. PMID: 22455317 Free PMC article.
-
Differential cytokine gene expression profiles in the three pathological forms of sheep paratuberculosis.BMC Vet Res. 2007 Aug 14;3:18. doi: 10.1186/1746-6148-3-18. BMC Vet Res. 2007. PMID: 17697353 Free PMC article.
-
Development and Validation of a Novel ELISA for the Specific Detection of Antibodies against Mycobacterium avium Subspecies paratuberculosis Based on a Chimeric Polyprotein.Vet Med Int. 2021 Dec 29;2021:7336848. doi: 10.1155/2021/7336848. eCollection 2021. Vet Med Int. 2021. PMID: 35003619 Free PMC article.
-
Prostaglandin E2 Induction Suppresses the Th1 Immune Responses in Cattle with Johne's Disease.Infect Immun. 2018 Apr 23;86(5):e00910-17. doi: 10.1128/IAI.00910-17. Print 2018 May. Infect Immun. 2018. PMID: 29483289 Free PMC article.
References
-
- Appelberg, R. 1994. Protective role of interferon gamma, tumor necrosis factor alpha and interleukin-6 in Mycobacterium tuberculosis and M. avium infections. Immunobiology 191:520-525. - PubMed
-
- Bannantine, J. P., J. F. Huntley, E. Miltner, J. R. Stabel, and L. E. Bermudez. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 149:2061-2069. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

